Abstract
Background/Aims
Methods
Results
Conclusions
Notes
FINANCIAL SUPPORT
The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST
Cheon JH has been the Editor of Intestinal Research since 2013. However, he was not involved in the peer reviewer selection, evaluation, or decision of this article. No other potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION
Acquisition of data: Park YE, Kim JN, Lee NR, Cheon JH. Analysis and interpretation of data: Park YE. Drafting of the manuscript: Park YE. Study concept and design: Kim JN, Lee NR, Park Y, Park SJ, Kim TI, Kim WH, Cheon JH. Critical revision of the manuscript for important intellectual content: Park Y, Park SJ, Kim TI, Kim WH, Cheon JH. All authors approved the final version of the article, including the authorship list.
Supplementary Materials
Supplementary Table 1.
REFERENCES
Fig. 1.
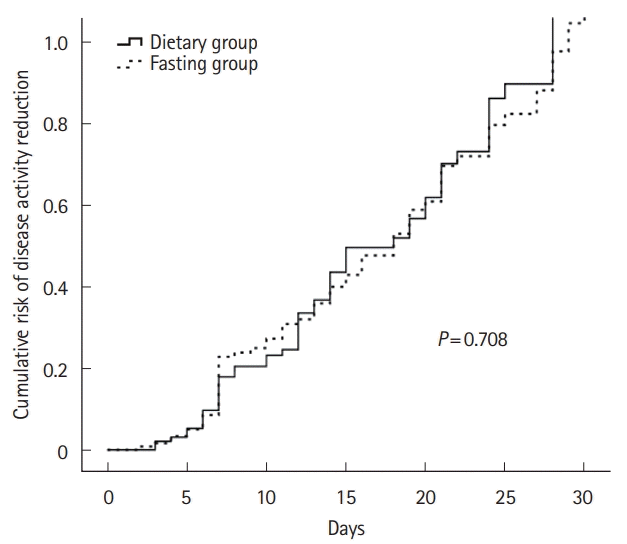
Table 1.
| Variable | Total (n = 222) | Fasting group (n = 124)a | Dietary group (n = 98)b | P-valuec |
|---|---|---|---|---|
| Male sex | 107 (48.2) | 60 (48.4) | 47 (48.0) | 0.949 |
| Age at admission (yr) | 40 (27–51) | 39 (25–49) | 40 (31–52) | 0.296 |
| Admission days | 9 (5–15) | 9 (5–15) | 8 (5–14) | 0.901 |
| Process of admission | ||||
| Emergency room | 100 (45.0) | 79 (63.7) | 21 (21.4) | < 0.001 |
| Outpatient clinic | 122 (55.0) | 45 (36.3) | 77 (78.6) | < 0.001 |
| Reasons for admission | 0.029 | |||
| Abdominal pain | 87 (39.2) | 54 (43.5) | 33 (33.7) | |
| GI bleeding | 26 (11.7) | 19 (15.3) | 7 (7.1) | |
| Fever | 16 (7.2) | 7 (5.6) | 9 (9.2) | |
| Diarrhea | 17 (7.7) | 11 (8.9) | 6 (6.1) | |
| Screening or work-up | 21 (9.5) | 9 (7.3) | 12 (12.2) | |
| General weakness | 29 (13.1) | 16 (12.9) | 13 (13.3) | |
| Othersd | 26 (11.7) | 8 (6.5) | 18 (18.4) | |
| Body weight (kg) | 55.0 (48.0–61.0) | 55.0 (50.3–62.0) | 52.0 (47.0–60.0) | 0.685 |
| BMI (kg/m2) | 20.1 (18.0–22.5) | 20.3 (18.3–22.5) | 19.8 (17.6–22.1) | 0.290 |
| Type of IBD | 0.242 | |||
| UC | 75 (33.8) | 36 (29.0) | 39 (39.8) | |
| CD | 82 (36.9) | 49 (39.5) | 33 (33.7) | |
| Intestinal Behçet’s disease | 65 (29.3) | 39 (31.5) | 26 (26.5) | |
| Consultation with the nutritional team | 65 (29.3) | 39 (31.5) | 26 (26.5) | 0.424 |
| Nutritional status by SNSI | 0.709 | |||
| Low risk of malnutrition | 158 (71.2) | 87 (70.2) | 71 (72.4) | |
| High risk of malnutrition | 64 (28.8) | 37 (29.8) | 27 (27.6) | |
| Change in diet prescription | 151 (68.0) | 119 (96.0) | 32 (32.7) | < 0.001 |
| Medications | ||||
| 5-ASA | 195 (87.8) | 111 (89.5) | 84 (85.7) | 0.389 |
| Steroids | 108 (48.6) | 58 (46.8) | 50 (51.0) | 0.530 |
| Immunomodulators | 89 (40.1) | 48 (38.7) | 41 (41.8) | 0.637 |
| Methotrexate | 18 (8.1) | 8 (6.5) | 10 (10.2) | 0.309 |
| Anti-TNF agents | 48 (21.6) | 21 (16.9) | 27 (27.6) | 0.056 |
| Total parenteral nutrition | 191 (86.0) | 113 (91.1) | 78 (79.6) | 0.014 |
| Enteral nutrition | 29 (13.1) | 19 (15.3) | 10 (10.2) | 0.261 |
| Previous bowel operation | 97 (43.7) | 56 (45.2) | 41 (41.8) | 0.620 |
| Underlying disease | ||||
| Hypertension | 19 (8.6) | 11 (8.9) | 8 (8.2) | 0.852 |
| Diabetes | 11 (5.0) | 5 (4.0) | 6 (6.1) | 0.476 |
| Tuberculosis | 25 (11.3) | 13 (10.5) | 12 (12.2) | 0.680 |
| Hematologic disorder | 37 (16.7) | 16 (12.9) | 21 (21.4) | 0.091 |
Table 2.
| Variable | Total (n = 222) | Fasting group (n = 124)a | Dietary group (n = 98)b | P-valuec |
|---|---|---|---|---|
| Laboratory findings | ||||
| Hemoglobin (g/dL) | 11.6 (10.0–13.6) | 12.0 (10.0–14.0) | 11.1 (10.0–13.0) | 0.174 |
| Initial ESR (mm/hr) | 50.5 (26.0–83.8) | 48.0 (22.0–84.5) | 52.0 (33.0–83.0) | 0.525 |
| Follow-up ESR (mm/hr) | 33.0 (15.3–59.0) | 10.0 (7.0–23.0) | 36.0 (17.5–58.5) | 0.562 |
| Initial CRP (mg/L) | 30.5 (5.7–103.7) | 23.5 (3.8–104.9) | 33.6 (9.0–103.9) | 0.754 |
| Follow-up CRP (mg/L) | 6.3 (1.4–23.4) | 6.4 (1.2–22.9) | 6.1 (1.7–25.8) | 0.296 |
| Initial albumin (g/dL) | 3.6 (3.0–4.0) | 3.6 (3.0–4.0) | 3.6 (3.0–4.0) | 0.908 |
| Follow-up albumin (g/dL) | 3.4 (2.8–4.0) | 3.5 (2.9–4.0) | 3.2 (2.5–3.9) | 0.002 |
| Disease activity | ||||
| UC | ||||
| Partial Mayo score | 6.0 (4.0–7.0) | 5.5 (4.0–8.3) | 6.0 (3.5–7.0) | 0.685 |
| Mayo score | 11.0 (8.0–13.0) | 11.5 (9.8–14.3) | 10.0 (7.0–12.3) | 0.064 |
| CD | 322.0 (236.0–425.0) | 308.0 (227.5–399.5) | 353.0 (281.5–461.0) | 0.065 |
| Intestinal Behçet’s disease | 90.0 (50.0–130.0) | 80.0 (50.0–120.0) | 80.0 (50.0–120.0) | 0.690 |
| Follow-up disease activity | ||||
| UC | ||||
| Partial Mayo score | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | 3.0 (2.0–5.5) | 0.953 |
| Mayo score | 6.0 (4.0–7.8) | 6.0 (4.3–8.0) | 4.5 (3.0–6.8) | 0.155 |
| CD | 320.5 (257.0–375.3) | 319.0 (286.5–393.5) | 322.0 (236.0–365.5) | 0.248 |
| Intestinal Behçet’s disease | 50.0 (25.0–80.0) | 55.0 (27.5–105.0) | 40.0 (20.0–60.0) | 0.239 |
| DAI reduction | 149 (67.1) | 82 (66.1) | 67 (68.4) | 0.724 |
| Readmission | 123 (55.4) | 70 (56.5) | 53 (54.1) | 0.724 |
Table 3.
| Variable |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| P-value | HR (95% CI) | P-value | Adjusted HR (95% CI) | |
| Male sex | 0.024 | 0.681 (0.488–0.950) | 0.044 | 0.661 (0.441–0.990) |
| Age at admission (yr) | 0.595 | 1.003 (0.992–1.014) | 0.200 | 0.990 (0.975–1.005) |
| Hospital stay (day) | 0.838 | 1.001 (0.989–1.014) | ||
| Diet prescription | ||||
| Dietary group | 1 (reference) | 1 (reference) | ||
| Fasting group | 0.825 | 0.964 (0.697–1.334) | 0.111 | 1.376 (0.929–2.039) |
| Body weight (kg) at admission | 0.078 | 0.985 (0.968–1.002) | 0.604 | 0.994 (0.974–1.016) |
| BMI (kg/m2) | 0.917 | 0.997 (0.949–1.048) | ||
| Process of admission | ||||
| Outpatient clinic | 1 (reference) | 1 (reference) | ||
| Emergency room | 0.025 | 0.684 (0.491–0.954) | 0.023 | 0.638 (0.434–0.939) |
| Type of IBD | ||||
| UC | 1 (reference) | 1 (reference) | ||
| CD | < 0.001 | 0.432 (0.290–0.644) | 0.067 | 0.574 (0.317–1.040) |
| Intestinal Behçet’s disease | 0.001 | 0.498 (0.331–0.748) | 0.001 | 0.397 (0.233–0.676) |
| Underlying disease | ||||
| Hypertension | 0.845 | 1.061 (0.587–1.917) | ||
| Diabetes | 0.746 | 0.889 (0.435–1.816) | ||
| Hematologic disorder | 0.774 | 0.936 (0.593–1.475) | ||
| Laboratory findings | ||||
| Hemoglobin (g/dL) | 0.004 | 0.908 (0.851–0.969) | 0.045 | 0.906 (0.824–0.998) |
| Albumin (g/dL) | 0.009 | 0.739 (0.589–0.927) | 0.594 | 0.912 (0.652–1.277) |
| ESR (mm/hr) | 0.363 | 1.002 (0.997–1.008) | 0.452 | 1.003 (0.995–1.011) |
| CRP (mg/L) | 0.392 | 0.999 (0.997–1.001) | 0.148 | 0.998 (0.995–1.001) |
| Medications | ||||
| 5-ASA | 0.185 | 1.451 (0.836–2.518) | 0.151 | 1.597 (0.843–3.025) |
| Corticosteroids | < 0.001 | 2.116 (1.507–2.970) | < 0.001 | 2.445 (1.506–3.969) |
| Immunomodulators | 0.219 | 0.811 (0.581–1.132) | 0.861 | 0.964 (0.637–1.459) |
| Anti-TNF agents | 0.590 | 0.893 (0.590–1.350) | 0.263 | 0.745 (0.445–1.247) |
| Othersa | 0.437 | 1.230 (0.730–2.072) | 0.554 | 1.229 (0.621–2.430) |
| Nutritional support | - | - | ||
| TPN | 0.422 | 0.833 (0.533–1.302) | ||
| EN | 0.719 | 0.915 (0.564–1.485) | ||
Table 4.
| Variable |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| p-value | OR (95% CI) | p-value | Adjusted OR (95% CI) | |
| Male sex | 0.160 | 0.674 (0.388-1.169) | 0.554 | 1.249 (0.597-2.612) |
| Age at admission (yr) | 0.002 | 1.029 (1.010-1.049) | 0.119 | 1.021 (0.995-1.048) |
| Hospital stay (day) | 0.211 | 1.015 (0.992-1.039) | ||
| Diet prescription | ||||
| Dietary group | 1 (reference) | 1 (reference) | ||
| Fasting group | 0.946 | 0.981 (0.566-1.701) | 0.620 | 1.201 (0.583-2.475) |
| Body weight (kg) at admission | 0.007 | 0.960 (0.931-0.989) | 0.163 | 0.972 (0.934-1.012) |
| BMI (kg/m2) | 0.306 | 0.955 (0.874-1.043) | ||
| Process of admission | ||||
| Out-patients clinic | 1 (reference) | 1 (reference) | ||
| Emergency room | 0.677 | 0.890 (0.513-1.543) | 0.598 | 0.823 (0.398-1.699) |
| Type of IBD | ||||
| UC | 1 (reference) | 1 (reference) | ||
| CD | 0.727 | 0.883 (0.440-1.773) | 0.627 | 0.771 (0.270-2.203) |
| Intestinal Behçet’s disease | 0.001 | 3.183 (1.583-6.402) | 0.012 | 3.263 (1.303-8.171) |
| Lab findings | ||||
| Hemoglobin (g/dL) | < 0.001 | 0.784 (0.694-0.886) | 0.044 | 0.841 (0.711-0.995) |
| Albumin (g/dL) | 0.007 | 0.576 (0.385-0.863) | 0.864 | 1.056 (0.564-1.980) |
| ESR (mm/hr) | 0.020 | 1.010 (1.002-1.019) | 0.705 | 1.002 (0.990-1.015) |
| CRP (mg/L) | 0.018 | 1.004 (1.001-1.007) | 0.769 | 0.999 (0.995-1.004) |
| Medications | ||||
| 5-ASA | 0.949 | 0.973 (0.423-2.241) | 0.431 | 1.534 (0.529-4.447) |
| Corticosteroids | 0.469 | 1.224 (0.708-2.115) | 0.233 | 0.626 (0.290-1.352) |
| Immunomodulators | 0.035 | 0.537 (0.302-0.956) | 0.700 | 0.863 (0.409-1.822) |
| Anti-TNF agents | 0.239 | 1.478 (0.771-2.832) | 0.345 | 1.520 (0.637-3.627) |
| Othersa | 0.170 | 1.857 (0.767-4.499) | 0.468 | 1.618 (0.441-5.937) |
| Nutritional support | - | - | ||
| TPN | 0.782 | 0.895 (0.410-1.955) | ||
| EN | 0.558 | 1.269 (0.573-2.810) | ||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download