This article has been
cited by other articles in ScienceCentral.
Inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis, is a chronic and inappropriate inflammatory disorder of the intestine. It is considered that interactions between genetic factors, microbiota, and host immune system are involved in the pathogenesis of IBD. Particularly, recent studies have shown that metabolites from intestinal microbes or host modulate the immune system in colitis [
1]. This study focused on macrophages and the role of succinate as an anti-inflammatory metabolite in IBD. Macrophages exist in almost all organs and are responsible for the first line of antimicrobial defense [
1]. The macrophages can be divided into pro-inflammatory M1 macrophages, called “classical macrophage,” and anti-inflammatory M2 macrophages, called “alternative macrophage.” It was reported that switching between M1 and M2 macrophages occurs through metabolic changes. In M2 macrophages, the succinate is oxidized to fumarate, resulting in electron transport chain flow that provides energy for adenosine triphosphate synthase. On the other hand, reverse electron transport occurs and succinate is accumulated in M1 macrophages [
2,
3]. If the polarization of macrophages is not properly regulated, this increases the proportion of M1 macrophages and can affect disease development such as IBD [
4].
Although there is a prior study using trinitrobenzene sulfonic acid-induced colitis and focusing on succinate receptor 1 (SUCNR1) and fibrosis [
5], it is not yet clear whether succinate directly affects macrophage activity in colitis. Wang et al. [
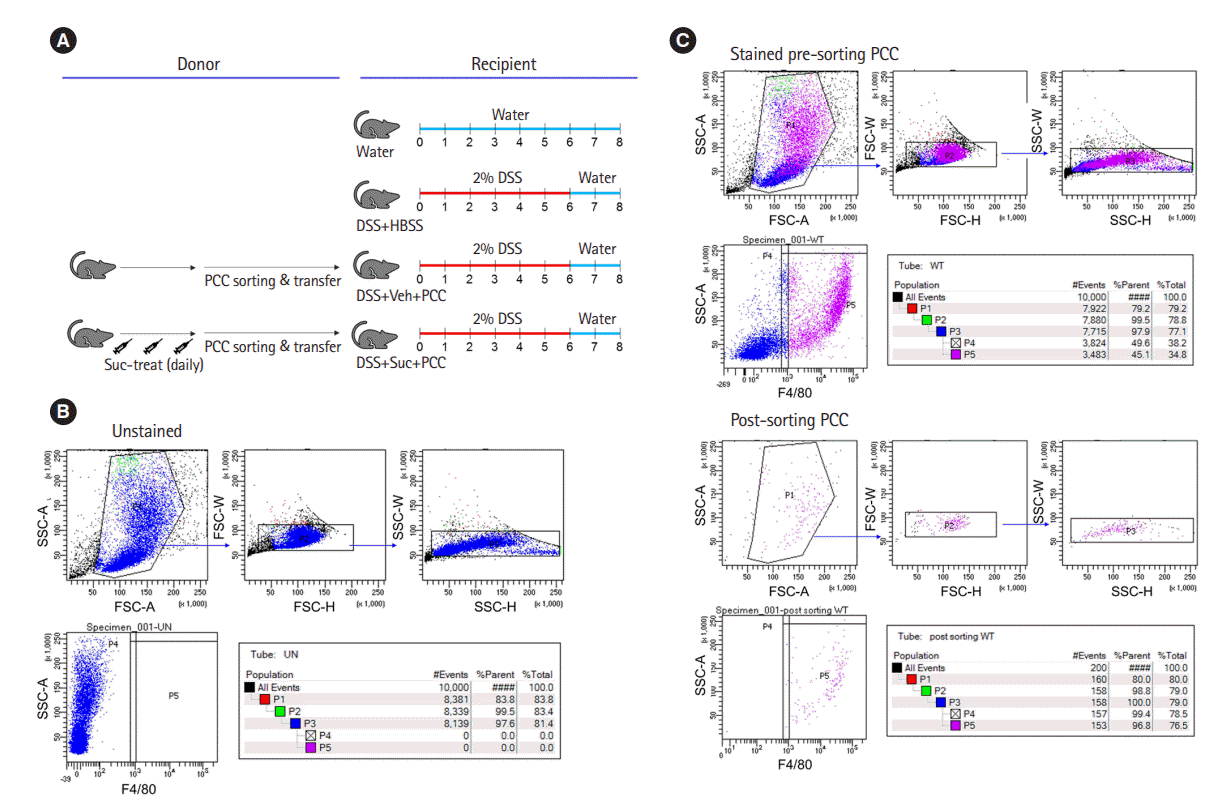
6] showed macrophages from peritoneal cavity cells (PCCs) affect experimental colitis. Hence, to identify the direct effect of succinate on macrophages, we performed a transfer experiment to find out the effects of succinate on macrophages in a dextran sulfate sodium (DSS) (MP Biomedicals, Solon, OH, USA)-induced colitis model (
Fig. 1A). All experiments using animals were reviewed and approved by the Institutional Animal Care and Use Committee of Yonsei University Severance Hospital (IACUC No: 2018-0227), and all methods were performed in accordance with relevant guidelines and regulations. Eight-weekold male C57BL/6 mice were maintained in 12 hours light/12 hours dark cycle at 22°C under specific pathogen‐free conditions and used as donors of peritoneal macrophages. The following groups of mice were used as donors: injected vehicle (Veh) (n = 4); injected succinate (Suc) (n = 4). F4/80
+ peritoneal macrophages were isolated using BD FACS Aria II (BD Biosciences, San Jose, CA, USA) from the collected peritoneal cells of mice which were then intraperitoneally injected with 40 mM succinate or not for 3 days (
Fig. 1B and
C). Isolated F4/80
+ cells of 2 × 10
5 were intraperitoneally injected to recipient mice in 200 μL Hanks’ balanced salt solution (HBSS; Thermo Fisher Scientific, Inc., Waltham, MA, USA) (day 0) and the mice were sacrificed after 2% (wt/vol) DSS in drinking water until day 6 followed by normal water administrated for 2 days. Recipient groups were as follows: water, administered with normal water (n = 3); DSS+HBSS, administered with HBSS (n = 6); DSS+VehPCC, recipients of PCC (n = 4); DSS+Suc-PCC, recipients of PCC treated succinate (n = 4). To estimate the severity of colitis, body weight, and disease activity index (DAI) were checked daily. The DAI scores, which ranged from 0 to 4, were calculated using the following parameters: body weight loss (0, none; 1, 1%–5%; 2, 5%–10%; 3, 10%–20%; 4, > 20%), stool consistency (0, negative; 1 and 2, loose; 3 and 4, diarrhea), and bleeding (0, absence; 1 and 2, slightly bleeding; 3 and 4, bloody) [
7]. The calculated formula was as follows: DAI = (body weight loss score)+ (stool consistency score)+(bleeding score). The colon length was measured between the ileocecal junction and the proximal rectum.
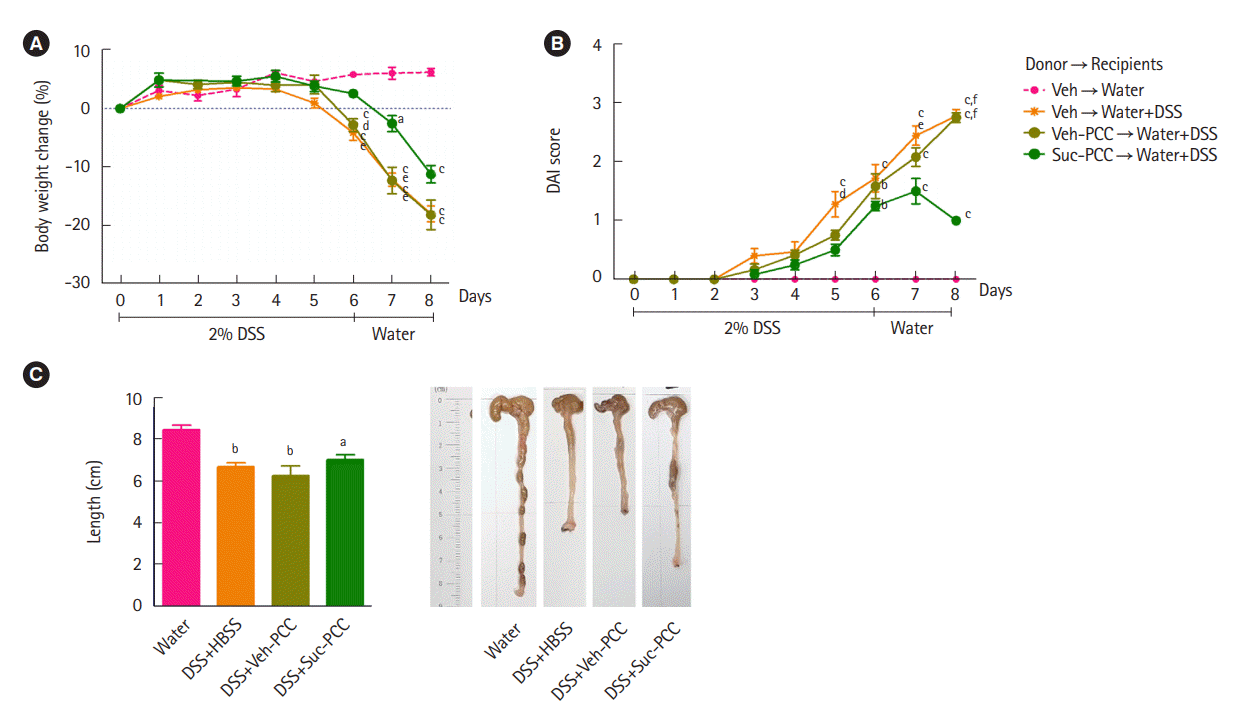
DSS+Suc-PCC mice showed the attenuated body weight loss (
Fig. 2A), DAI (
Fig. 2B), and a colon length shortening (
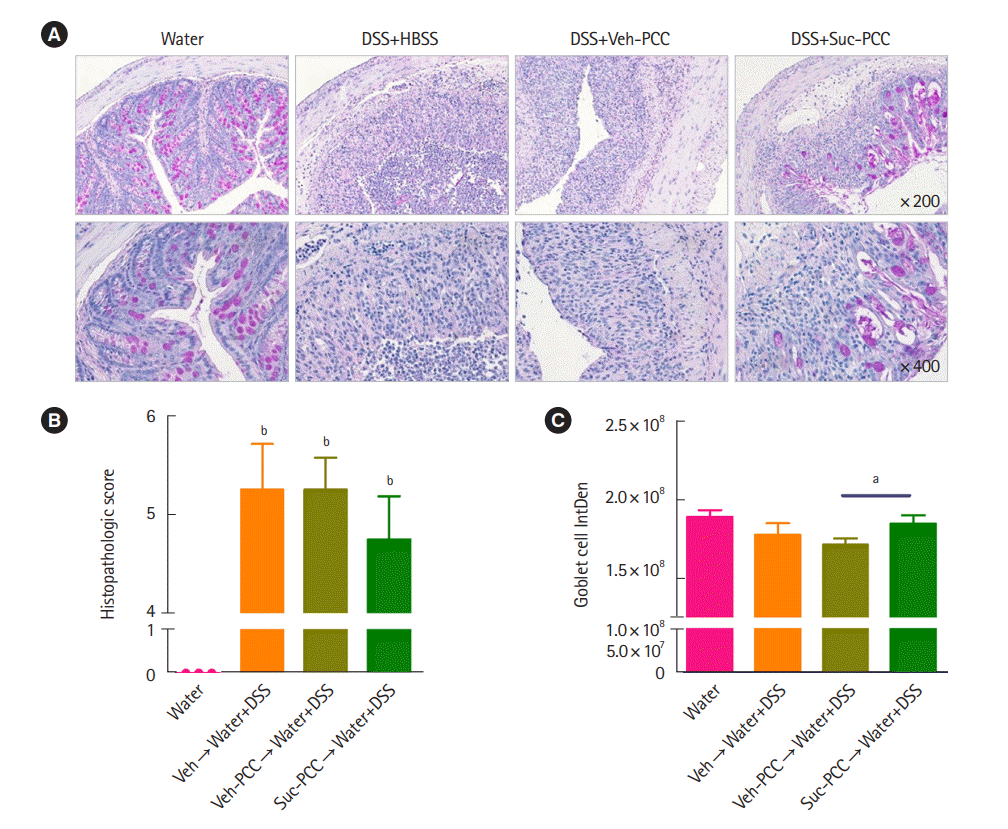
Fig. 2C) compared with DSS+Veh-PCC mice. For histological analysis, segments of distal colons were embedded in paraffin and stained with periodic acid‐Schiff to observe goblet cells and mucins. We found that mucin levels were significantly increased in succinate-treated PCC group compared with DSS+Veh-PCC group (
Fig. 3). Taken together, these results suggest that succinate-treated PCC attenuates DSS-induced colitis.
Although some researches on succinate have mainly described succinate as a pro-inflammatory factor in many inflammatory diseases [
8], there are contradictory studies that succinate acts as an anti-inflammatory factor [
9,
10]. The succinate secreted by the cancer cells into their microenvironment induces polarization into tumor-associated macrophages through SUCNR1 signaling of macrophages [
9]. Besides, another study showed that SUCNR1 on macrophages plays a role in inducing antiinflammatory phenotype and limiting inflammation [
10]. These controversial results showing pro- and anti-inflammatory effects of succinate might be due to the complexity of the immune system. In this study, we addressed the protective function of succinate in macrophages.
It is well known that succinate dehydrogenase is impaired in inflammatory conditions, and then succinate is accumulated. Further studies investigating the effects of succinate on various macrophages types, using cell lines and primary cells from Sucnr1-/- mice would be warranted.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download