Abstract
Background/Aims
Methods
Results
Notes
Conflict of Interest
Suzuki Y reports grants and personal fees from AbbVie GK, and personal fees from Eisai Co. Ltd., during the conduct of the study; and grants and personal fees from AbbVie GK, EA Pharma Co. Ltd., JIMRO Co. Ltd, KISSEI Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, and Nippon Kayaku Co. Ltd; personal fees from Eisai Co. Ltd., Gilead Sciences, Inc., Janssen Pharmaceutical K.K., KYORIN Pharmaceutical Co. Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Company, and Zeria Pharmaceutical Co. Ltd., outside the submitted work. Hagiwara T is an employee of AbbVie GK and reports stock/stock options of AbbVie GK outside the submitted work. Kobayashi M, Morita K, and Shimamoto T are employees of AbbVie GK. Hibi T reports grants and personal fees from AbbVie GK, and personal fees from EA Pharma Co. Ltd., during the conduct of the study and outside the submitted work.
Hibi T is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
Conceptualization: Suzuki Y, Hagiwara T. Data curation: Morita K. Formal analysis: Suzuki Y, Hagiwara T, Kobayashi M, Hibi T. Methodology: Shimamoto T. Supervision: Hibi T. Writing - original draft: all authors. Writing - review & editing: all authors. Approval of final manuscript: all authors.
Others
This study was sponsored by AbbVie GK and Eisai Co., Ltd. The sponsors participated in the study design, data collection, analysis and interpretation of data, and writing, reviewing, and approving the publication. The standard operation procedure manual for data management and transformation was developed by A2 Healthcare Corporation and approved by the Post-Marketing Surveillance group in AbbVie. Medical writing support, including creating tables based on detailed instructions from the authors, collating author comments, copyediting, fact-checking, and referencing, was provided by Dr Deepali Garg and Frances Gambling of Cactus Communications. The above-mentioned support was funded by the sponsors.
Supplementary Material
Supplementary Table 1.
REFERENCES
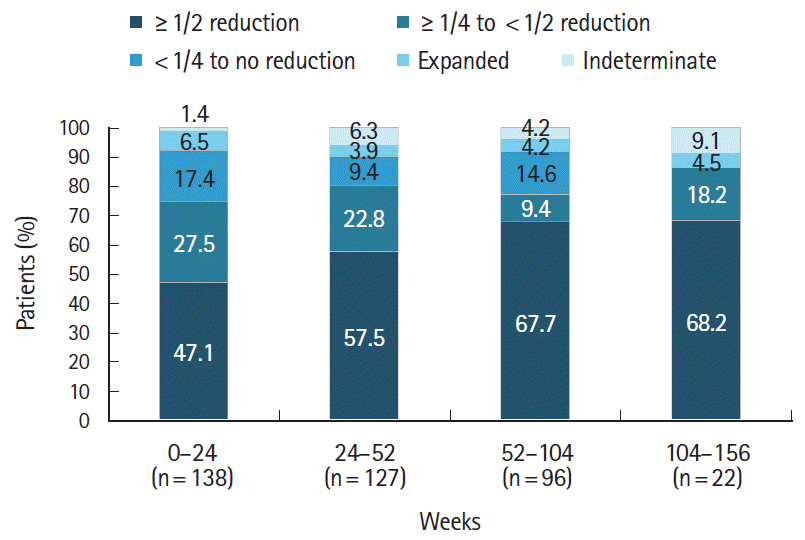
Fig. 1.
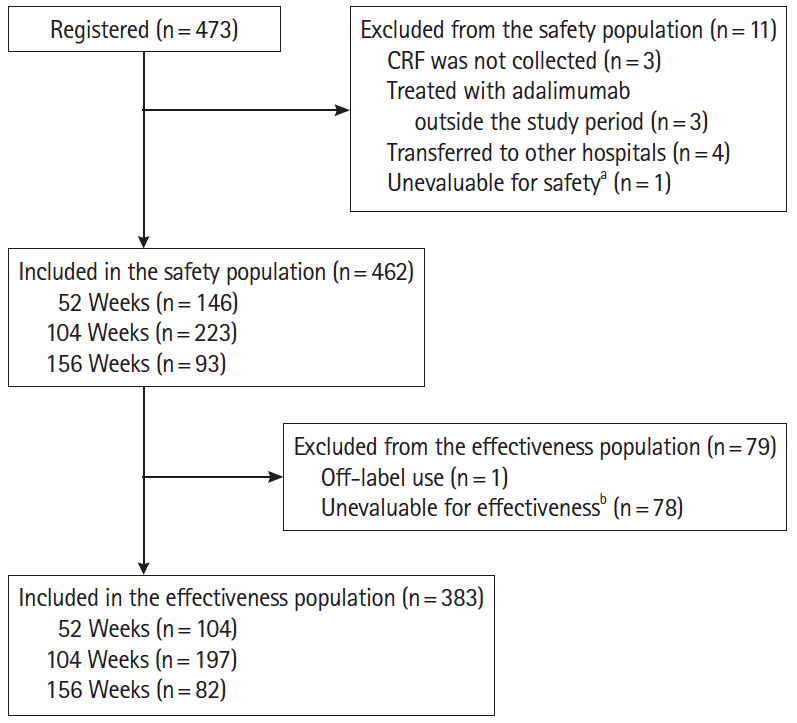
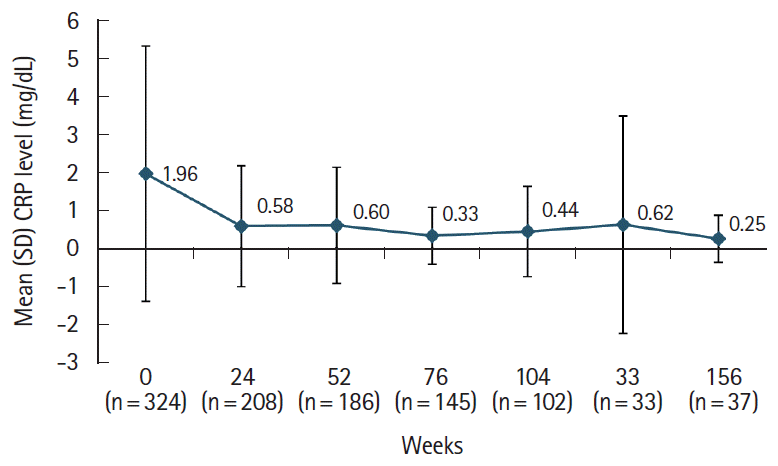
Fig. 2.
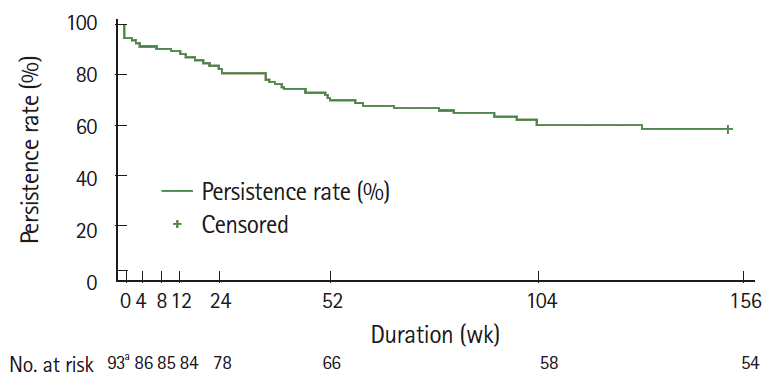
Fig. 3.
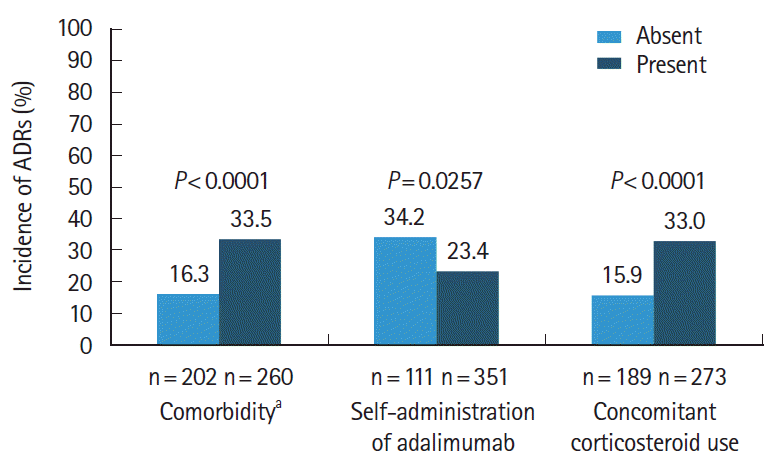
Fig. 4.
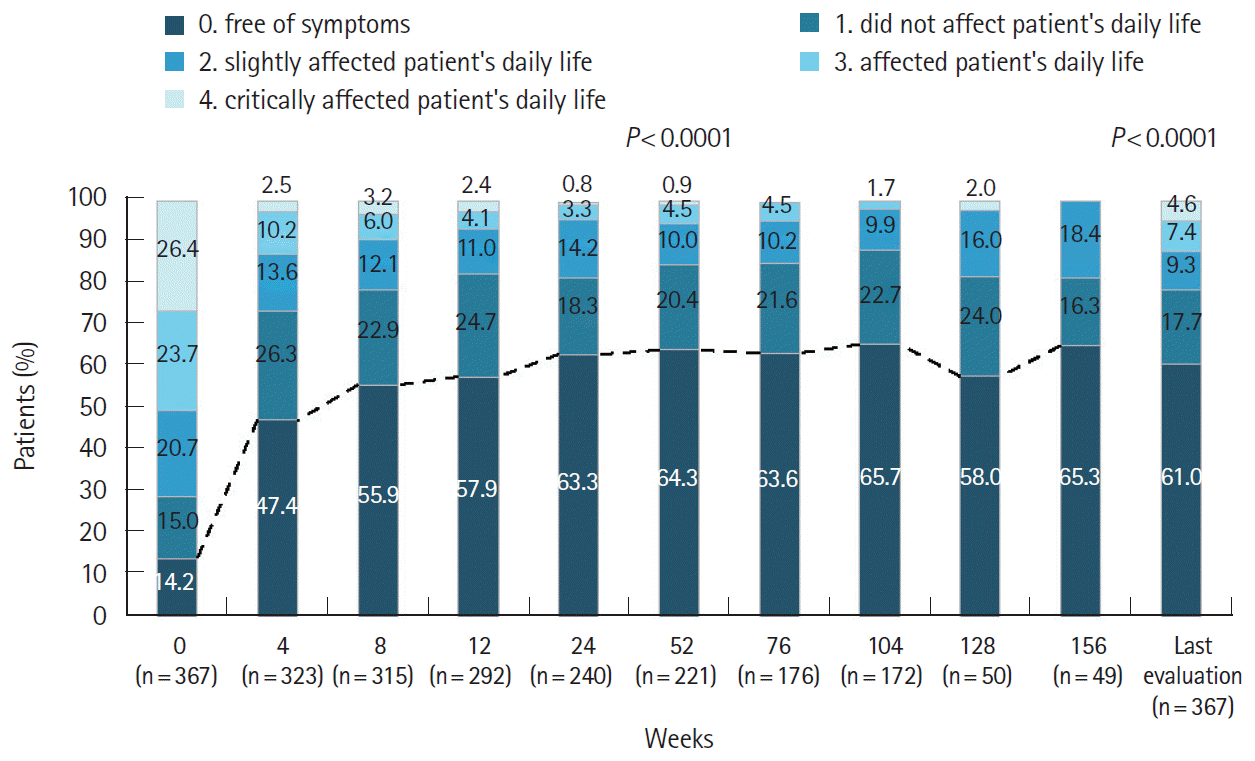
Table 1.
| Characteristic | Value (n = 462) |
|---|---|
| Female sex | 225 (48.7) |
| Age (yr) | 46.3 ± 17.2 |
| Body weight (kg) | 56.2 ± 12.6 |
| BMI (kg/m2) | 21.3 ± 3.7 |
| Disease duration (yr) | 7.6 ± 8.3 |
| Diagnostic category of BDa | |
| Complete | 25 (5.4) |
| Incomplete | 233 (50.4) |
| Suspected | 177 (38.3) |
| Other | 27 (5.8) |
| Comorbidities (present) | 260 (56.3) |
| Past medical history (known) | 97 (21.0) |
| Self-administration (yes) | 351 (76.0) |
| TB test conducted | 370 (80.1) |
| Previous treatment (present) | 447 (96.8) |
| Biological agent useb | |
| Adalimumab | 24 (5.4) |
| Infliximab | 70 (15.7) |
| Others | 5 (1.1) |
| Non-biologic agent useb | |
| Aminosalicylates | 286 (64.0) |
| Corticosteroids | 320 (71.6) |
| Immunomodulators | 161 (36.0) |
| Antibiotics | 47 (10.5) |
| Others | 193 (43.2) |
| Concomitant drugs (present) | 445 (96.3) |
| Concomitant drugsc | |
| Aminosalicylates | 272 (61.1) |
| Corticosteroids | 273 (61.3) |
| Tacrolimus/cyclosporine | 31 (7.0) |
| Azathioprine/6-mercaptopurine | 142 (31.9) |
| Enteral nutrients | 27 (6.1) |
| Colchicine | 170 (38.2) |
| Others | 362 (81.3) |
a Per Japanese diagnostic criteria [5]: complete, 4 major symptoms; incomplete, 3 major/2 major and 2 minor symptoms/typical ocular symptoms and one other major symptom/2 minor symptoms; suspected, partial major symptoms not satisfying the conditions of abortive type, and recurrence or exacerbation of typical minor symptoms; other, no major symptoms.
Table 2.
| Safety event |
Any AE/ADR |
Serious AE/ADR |
||
|---|---|---|---|---|
| Total | Event ratea | Total | Event ratea | |
| AEs | 165 (35.71) | 46.98 | 75 (16.23) | 15.37 |
| ADRs | 120 (25.97) | 30.74 | 51 (11.04) | 10.44 |
| ADRs of interest | ||||
| Infections | 47 (10.17) | 11.02 | 18 (3.90) | 3.92 |
| Injection site reactions | 5 (1.08) | 0.73 | 0 | 0 |
| Tuberculosis | 3 (0.65)b | 0.44 | 1 (0.22) | 0.15 |
| Interstitial pneumonia | 2 (0.43) | 0.29 | 1 (0.22) | 0.15 |
| Malignancy | 1 (0.22) | 0.15 | 1 (0.22) | 0.15 |
| Autoimmune disease | 1 (0.22) | 0.15 | 1 (0.22) | 0.15 |
| Pancytopenia | 1 (0.22) | 0.15 | 1 (0.22) | 0.15 |
| Demyelinating disease | 0 | - | 0 | - |
| Cardiac failure congestive | 0 | - | 0 | - |
Table 3.
Table 4.
| Observation period | No. of total patientsa | Patients with symptoms |
|---|---|---|
| Oral aphthous ulcers | ||
| Week 0 | 183 | 183 (100.0) |
| Week 52 | 113 | 29 (25.7) |
| Week 104 | 85 | 26 (30.6) |
| Week 156 | 17 | 5 (29.4) |
| At last evaluation time point | 183 | 62 (33.9) |
| Skin symptoms | ||
| Week 0 | 96 | 96 (100.0) |
| Week 52 | 67 | 11 (16.4) |
| Week 104 | 43 | 9 (20.9) |
| Week 156 | 13 | 0 |
| At last evaluation time point | 96 | 23 (24.0) |
| Eye symptoms | ||
| Week 0 | 4 | 4 (100.0) |
| Week 52 | 4 | 1 (25.0) |
| Week 104 | 4 | 0 |
| Week 156 | 1 | 0 |
| At last evaluation time point | 4 | 0 |
| Genital ulcer | ||
| Week 0 | 51 | 51 (100.0) |
| Week 52 | 35 | 8 (22.9) |
| Week 104 | 18 | 4 (22.2) |
| Week 156 | 6 | 1 (16.7) |
| At last evaluation time point | 51 | 13 (25.5) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download