1. Morita N, Toki S, Hirohashi T, et al. Incidence and prevalence of inflammatory bowel disease in Japan: nationwide epidemiological survey during the year 1991. J Gastroenterol. 1995; 30(Suppl 8):1–4.
2. Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol. 2009; 44:659–665.

3. Sood A, Midha V, Sood N, Bhatia AS, Avasthi G. Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut. 2003; 52:1587–1590.

4. Yang SK, Hong WS, Min YI, et al. Incidence and prevalence of ulcerative colitis in the Songpa-Kangdong district, Seoul, Korea, 1986-1997. J Gastroenterol Hepatol. 2000; 15:1037–1042.

5. Lai CL, Wu PC, Wong KL, Lok ASF. Clinical features of ulcerative proctocolitis in Hong Kong Chinese: a review of three decades. Am J Proctol Gastroenterol Colon Rectal Surg. 1985; 36:14–19.
6. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008; 14:542–549.

7. Makharia GK, Ramakrishna BS, Abraham P, et al. Survey of inflammatory bowel diseases in India. Indian J Gastroenterol. 2012; 31:299–306.

8. Munkholm P. Crohn’s disease: occurrence, course and prognosis: an epidemiologic cohort-study. Dan Med Bull. 1997; 44:287–302.
9. Langholz E. Ulcerative colitis: an epidemiological study based on a regional inception cohort, with special reference to disease course and prognosis. Dan Med Bull. 1999; 46:400–415.
10. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006; 55:749–753.

11. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011; 17:1314–1321.

12. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976; 70:439–444.
13. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987; 317:1625–1629.

14. Kedia S, Ahuja V. Epidemiology of inflammatory bowel disease in India: the great shift East. Inflamm Intest Dis. 2017; 2:102–115.

15. Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004; 126:1504–1517.

16. Moum B, Vatn MH, Ekbom A, et al. Incidence of Crohn’s disease in four counties in southeastern Norway, 1990-93: a prospective population-based study. The Inflammatory Bowel South-Eastern Norway (IBSEN) Study Group of Gastroenterologists. Scand J Gastroenterol. 1996; 31:355–361.

17. Moum B, Vatn MH, Ekbom A, et al. Incidence of ulcerative colitis and indeterminate colitis in four counties of southeastern Norway, 1990-93: a prospective population-based study. The Inflammatory Bowel South-Eastern Norway (IBSEN) Study Group of Gastroenterologists. Scand J Gastroenterol. 1996; 31:362–366.

18. Loftus EV Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn’s disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology. 1998; 114:1161–1168.

20. Solberg IC, Vatn MH, Høie O, et al. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007; 5:1430–1438.

21. Das K, Ghoshal UC, Dhali GK, Benjamin J, Ahuja V, Makharia GK. Crohn’s disease in India: a multicenter study from a country where tuberculosis is endemic. Dig Dis Sci. 2009; 54:1099–1107.

22. Kalaria R, Desai D, Abraham P, Joshi A, Gupta T, Shah S. Temporal change in phenotypic behaviour in patients with Crohn’s disease: do Indian patients behave differently from Western and other Asian patients? J Crohns Colitis. 2016; 10:255–261.

23. Sood A, Midha V, Sood N, Bansal M. Long term results of use of azathioprine in patients with ulcerative colitis in India. World J Gastroenterol. 2006; 12:7332–7336.

24. Sood A, Midha V, Sood N, Avasthi G. Azathioprine versus sulfasalazine in maintenance of remission in severe ulcerative colitis. Indian J Gastroenterol. 2003; 22:79–81.
25. Sood A, Kaushal V, Midha V, Bhatia KL, Sood N, Malhotra V. The beneficial effect of azathioprine on maintenance of remission in severe ulcerative colitis. J Gastroenterol. 2002; 37:270–274.

26. Bopanna S, Ananthakrishnan AN, Kedia S, Yajnik V, Ahuja V. Risk of colorectal cancer in Asian patients with ulcerative colitis: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017; 2:269–276.

27. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–2917.

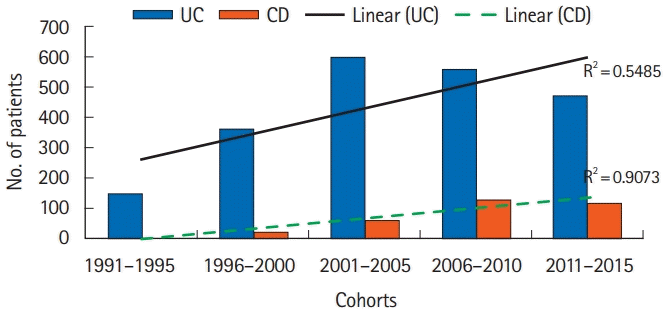
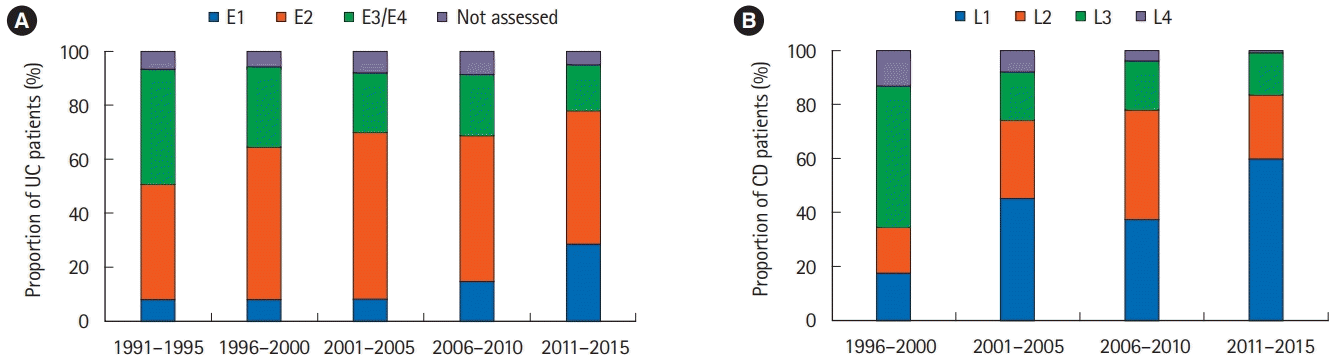
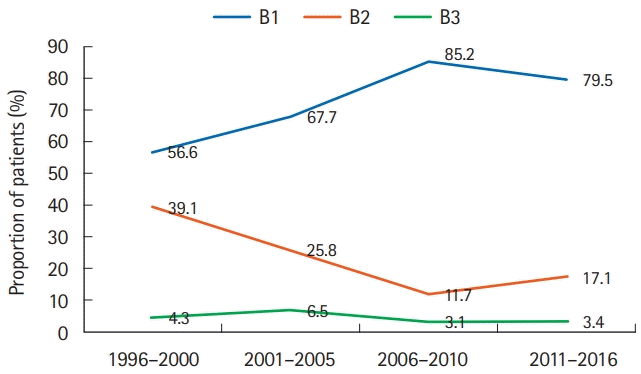
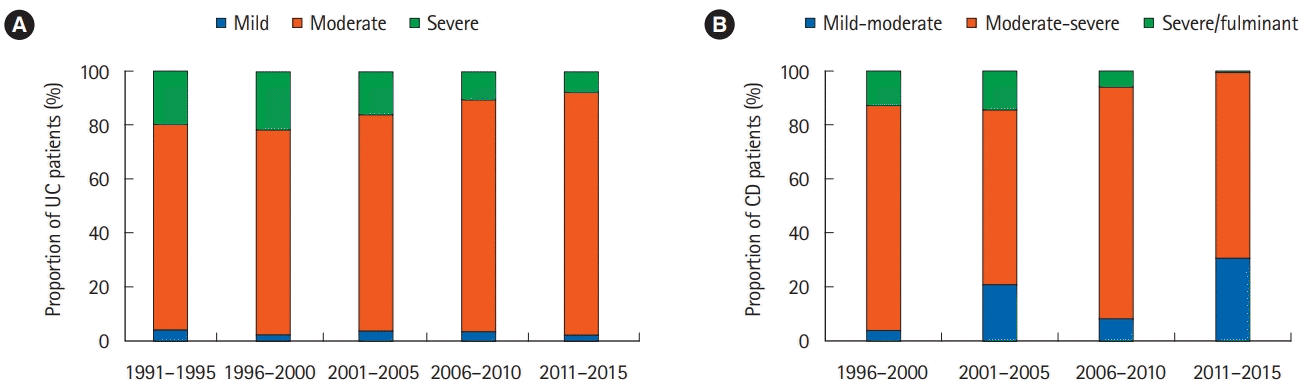




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download