Abstract
Patients with Crohn’s disease (CD) commonly develop bowel strictures, which may contain various degrees of inflammation and fibrosis. While predominantly inflammatory strictures may benefit from a medical anti-inflammatory treatment approach, fibrotic strictures would require endoscopic balloon dilation or surgery. Cross-sectional imaging surpasses endoscopy for characterization of stenotic segments and potentially may contribute to the optimal clinical management of these patients. This short review aims to discuss the potentialities and limitations of cross-sectional imaging techniques for assessing bowel fibrosis in patients with CD.
The development of an intestinal stricture in patients with CD represents an important event on the natural history of these patients that threatens an increased risk of surgery [1-3].
Up to 10% of patients with CD present with a stricture at diagnosis and up to half of patients will progress to a stricturing phenotype during their life [4,5].
The mechanisms by which strictures develop in CD are complex. The most accepted hypothesis is that excessive repair response to bowel inflammation causes a reduction in luminal diameter, which is dependent on both the pleiotropic actions of inflammatory mediators and the interplay of profibrotic genetic, cellular, and microbiota-related factors [6]. This interaction, therefore would explain the overlap between both inflammatory and fibrotic components reported on several publications [7-15].
The accurate determination of the extent of fibrosis in involved bowel segments is important for various reasons. In clinical practice, it is essential to differentiate between strictures that are predominantly due to fibrosis and those that are predominantly due to inflammation, because fibrotic strictures require endoscopic balloon dilation or surgery, but predominantly inflammatory strictures may benefit from anti-inflammatory treatment. This differentiation could also help us to understand the role of fibrosis in symptoms in the absence of inflammation. Finally, the accurate measurement of fibrosis is essential for the development and testing of drugs to prevent and treat strictures due to fibrosis [16]. Such drugs would be a great step forward in the management of CD, as currently available treatments all primarily aim to reduce inflammation rather than fibrosis [17].
Endoscopic biopsies are unable to measure the amount of fibrosis in the intestinal wall. Cross-sectional imaging modalities can identify strictures in both the small and large bowel, and given the transmural nature of CD, enable a more objective assessment including the differentiation between inflammation and fibrosis.
In recent years, various cross-sectional imaging techniques have been incorporated into daily practice and research to better characterize bowel strictures and to quantify the degree of fibrosis. Although the evidence on the utility of these techniques is still weak, in the near future, they may provide information critical for planning therapeutic management [18,19].
Cross-sectional imaging is highly accurate in detecting inflammatory lesions [18,19]. One key feature indicating the presence of active disease is mural hyperenhancement on a thickened bowel segment. When evidence of mural hyperenhancement, edema, or hypervascularity is lacking on cross-sectional imaging of the bowel, fibrosis is often assumed. However, fibrosis is closely linked to inflammation, and both components are frequently superimposed in stenotic segments [6]; therefore, standard imaging modalities might be unable to differentiate between fibrosis and inflammation [8-10,13]. Some studies based on conventional morphologic and/or non-dynamic contrast-enhanced imaging features on the bowel were unable to detect or quantify the amount of fibrosis in the bowel beyond identifying strictures with proximal dilation [7] and others reported conflicting results [9,11].
Evidence from more recent studies indicates that new imaging techniques are being developed including diffusion-weighted imaging, dynamic contrast-enhanced MRI, magnetization transfer (Fig. 1) or shear-wave and strain-wave ultrasound elastography (Fig. 2) promise to improve the quantification of bowel wall fibrosis [20]. Table 1 summarizes the main characteristics of novel imaging modalities that had been investigated to detect and quantify fibrosis in CD, whereas Table 2 provides the evidence obtained in studies testing the techniques against histopathology [9-11,13-15,21-25]. To date, all the evidence comes from single-center studies with relatively small samples. Before these noninvasive quantitative imaging biomarkers can be widely implemented, further multicenter large-scale studies are needed to establish cutoff values, test their reproducibility, and determine their degree of interobserver agreement.
Differentiation between fibrotic and inflammatory strictures remains a crucial challenge in the management of CD. There is currently no standardized approach to determine whether intestinal strictures are predominantly due to fibrosis or inflammation. The frequent superimposition between inflammatory and the fibrotic component in stenotic segments hampers its proper characterization. Traditional cross-sectional imaging modalities were unable to detect fibrosis, but recent studies using elastography and advanced MRI techniques such as diffusion-weighted imaging and magnetization transfer sequences have reported interesting findings that promise to improve the detection and grading of fibrosis in CD. Before these techniques can be fully incorporated into routine practice, however, further studies are necessary to establish cutoff values, test their reproducibility, and determine the interobserver agreement.
Notes
REFERENCES
1. Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013; 62:1072–1084.

2. Rieder F, Latella G, Magro F, et al. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J Crohns Colitis. 2016; 10:873–885.

3. Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016. Part 1: diagnosis and medical management. J Crohns Colitis. 2017; 11:3–25.

4. Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002; 8:244–250.

5. Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001; 49:777–782.

6. Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017; 152:340–350.

7. Chiorean MV, Sandrasegaran K, Saxena R, Maglinte DD, Nakeeb A, Johnson CS. Correlation of CT enteroclysis with surgical pathology in Crohn’s disease. Am J Gastroenterol. 2007; 102:2541–2550.

8. Adler J, Punglia DR, Dillman JR, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn’s disease. Inflamm Bowel Dis. 2012; 18:849–856.

9. Zappa M, Stefanescu C, Cazals-Hatem D, et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis. 2011; 17:984–993.

10. Rimola J, Planell N, Rodríguez S, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015; 110:432–440.

11. Punwani S, Rodriguez-Justo M, Bainbridge A, et al. Mural inflammation in Crohn disease: location-matched histologic validation of MR imaging features. Radiology. 2009; 252:712. 720.

12. Stidham RW, Xu J, Johnson LA, et al. Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn’s disease. Gastroenterology. 2011; 141:819–826.

13. Tielbeek JA, Ziech ML, Li Z, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol. 2014; 24:619–629.

14. Li XH, Mao R, Huang SY, et al. Characterization of degree of intestinal fibrosis in patients with Crohn disease by using magnetization transfer MR imaging. Radiology. 2018; 287:494–503.

15. Wagner M, Ko HM, Chatterji M, et al. Magnetic resonance imaging predicts histopathological composition of ileal Crohn’s disease. J Crohns Colitis. 2018; 12:718–729.

16. Rieder F, Bettenworth D, Ma C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn’s disease. Aliment Pharmacol Ther. 2018; 48:347–357.

17. Jairath V, Levesque BG, Vande Casteele N, et al. Evolving concepts in phases I and II drug development for Crohn’s disease. J Crohns Colitis. 2017; 11:246–255.

18. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019; 13:144–164.

19. Bruining DH, Zimmermann EM, Loftus EV Jr, et al. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Gastroenterology. 2018; 154:1172–1194.

20. Bettenworth D, Bokemeyer A, Baker M, et al. Assessment of Crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review. Gut. 2019; 68:1115–1126.

21. Wilkens R, Hagemann-Madsen RH, Peters DA, et al. Validity of contrast-enhanced ultrasonography and dynamic contrast-enhanced MR enterography in the assessment of transmural activity and fibrosis in Crohn’s disease. J Crohns Colitis. 2018; 12:48–56.

22. Dillman JR, Stidham RW, Higgins PD, et al. Ultrasound shear wave elastography helps discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens. J Ultrasound Med. 2014; 33:2115–2123.

23. Fraquelli M, Branchi F, Cribiù FM, et al. The role of ultrasound elasticity imaging in predicting ileal fibrosis in Crohn’s disease patients. Inflamm Bowel Dis. 2015; 21:2605–2612.

Fig. 1.
Images in a 57-year-old woman with CD with past history of ileocecal resection and new stricture on the neo-terminal ileum. Axial diffusion-weighted images with b=800 sec/mm2 (A) and hypointensity on corresponding apparent diffusion coefficient (ADC) map (ADC=923 mm2 /sec) (B) are shown in the same segment (arrow). Axial magnetization transfer (MT) imaging without (C) and with MT pulse and (D) demonstrate MT effect of neo-terminal ileum (arrow) to be similar to that of skeletal muscle.
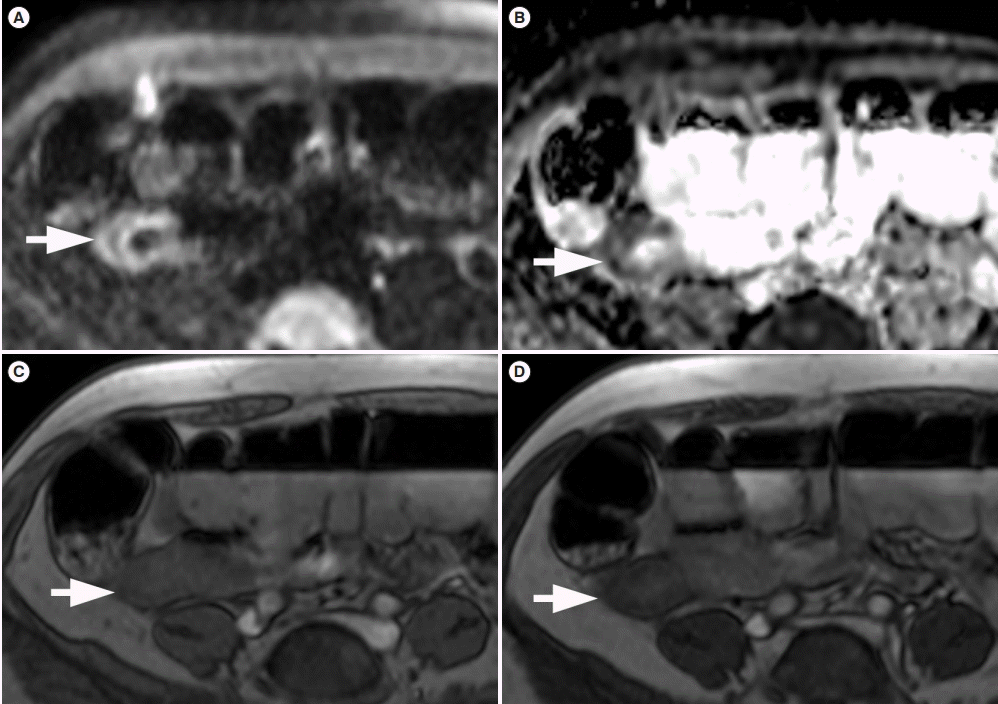
Fig. 2.
Real-time shear wave ultrasound image of stenotic bowel wall (left image) overlaying conventional ultrasound gray-scale images (right image) in a 38-year-old woman with CD. Multiple circular region of interest (ROI) are depicted over the bowel wall (T1–T3 and T4 [not seen on this image]) and in perienteric fat (R1). The quantitative data (average stiffness value in kPa and SD of each ROI, and ratio between bowel wall and fat) is automatically calculated. The color scale inside the box on the left image indicates the distribution of the measured elasticity within the area of interest. Ave, average.
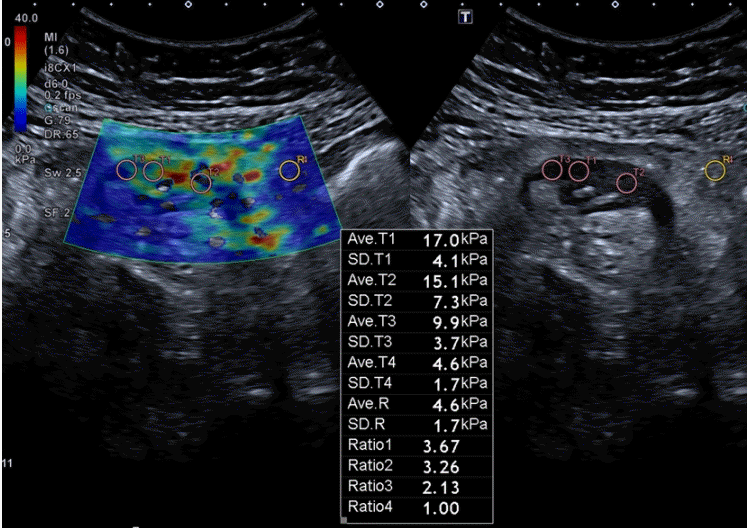
Table 1.
Main Novel Imaging Modalities and Their Main Characteristics
Table 2.
Overview of Current Evidence on the Use of Cross-Sectional Imaging Studies Assessing the Inflammatory and Fibrotic Components of CD Strictures Using Histopathology from Surgical Ressected Specimens as Reference Standard
| Author (year) | Technique | Nature of the study | Main clinically relevant findings | |
|---|---|---|---|---|
| Magnetic resonance | ||||
| Punwani et al. (2009) [11] | MRE (morphologic and enhancement features) | Single-center study | The study aimed to determine the association between MRI features of CD activity against a histopathologic reference. | |
| Prospective | Different MR features were correlated with inflammation was found in both studies including wall thickness, T2 hypersignal and layered enhancement of gadolinium. | |||
| Wall thickness was not correlated with the degree of fibrosis. | ||||
| Zappa et al. (2011) [9] | MRE (morphologic and enhancement features) | Single-center study | The study aimed to evaluate the value of MRI findings in CD in correlation with pathological scores of inflammation and fibrosis. | |
| Retrospective | Different MR features were correlated with inflammation including wall thickness, T2 hypersignal and layered enhancement of gadolinium. Layered enhancement was not associated with the degree of fibrosis. | |||
| Both inflammation and fibrosis scores were positively correlated (r = 0.63, P = 0.0001). | ||||
| Wilkens et al. (2018) [21] | MRE (morphologic and enhancement features) | Single-center study | Authors investigated the perfusion by MRE as objective marker to distinguishing between the inflammation and fibrosis. | |
| Prospective | Wall thickness correlated with the degree of inflammation. | |||
| No significant correlation between the severity of inflammation or fibrosis on histopathology, and mural enhancement (r = –0.13, P = 0.54 for inflammation and r = 0.41, P = 0.05 for fibrosis). | ||||
| Tielbeek et al. (2014) [13] | MRE with DWI and perfusion analysis of contrast injection | Single-center study | Different advanced MR techniques were applied before surgical resection, including diffusion- weighted image, perfusion analysis including time-intensity curves, and morphological assessment as well. | |
| Retrospective | Mural thickness, maximum intravenous contrast enhancement and the slope of increase after its injection correlated significantly with histological inflammation (r = 0.63, 0.41, 0.53, respectively; P< 0.05). | |||
| The quantification of diffusion-weighted imaging by mean of ADC correlated significantly with fibrosis (all P< 0.05). | ||||
| Rimola et al. (2015) [10] | MRE using DCE (gain of enhancement between early and late phases) | Single-center study | Different imaging acquisitions were obtained after gadolinium injection. The hypothesis of this study was that dense fibrotic typically show a slow enhancement of gadolinium contrast over time. | |
| Retrospective | Using percentage of enhancement gain between early and late phases after gadolinium injection, MRI is able to discriminate between mild–moderate and severe fibrosis deposition with a sensitivity of 0.94 and a specificity of 0.89. | |||
| T2 hypersignal (edema) has a high and significant predictive value for detecting severe inflammation. | ||||
| Li et al. (2018) [14] | MRE using MTR, ADC and gain of enhancement | Single-center study | MT sequence attempts to identify tissue with water linked to macromolecules (such as collagen). The study compared MT with diffusion and the gain of gadolinium enhancement. | |
| Prospective | Differentiating moderately to severely fibrotic bowel walls from those non-to-mildly fibrotic with an AUC of 0.919 (P = 0.000) for MT measurements; AUC of 0.747 (P = 0.001) for ADC; and AUC of 0.592 (P = 0.209) for the percentage of enhancement gain. | |||
| Repeated measurements | ||||
| Wagner et al. (2018) [15] | MRE using DWI | Single-center study | Authors hypothesized that muscular hypertrophy is an additional lesion that reduces bowel lumen in strictures and should be differentiated from fibrosis. Analyzing the bowel wall thickness on T2W (> 7.4 mm) had a sensitivity of 61% and a specificity of 89% to differentiate fibrosis from muscular hypertrophy. | |
| Retrospective | ||||
| Ultrasound | ||||
| Dillman et al. (2014) [22] | USE (SWE) | Single-center study | The study aimed to determine if US elastography could discriminate low- from high-grade fibrosis in the bowel. The authors used 2 different methods of SWE. | |
| Prospective | Significant correlation between shear wave speed and bowel fibrosis (P = 0.01). USE using 2 different methods of USE it is possible to differentiate low– from high–fibrosis score bowel segments with AUC of 0.77–0.91. No significant differences in USE in mean shear wave speed between high- and low-inflammation score bowel segments. | |||
| Fraquelli et al. (2015) [23] | USE (strain ratio) | Single-center study | The aim was to evaluate the feasibility of USE toward the assessment of ileal fibrosis in CD patients. USE strain ratio measurement was significantly correlated with the severity of bowel fibrosis 0.917 (95% CI, 0.788–1.000). | |
| Prospective | ||||
| Wilkens et al. (2018) [21] | Bowel US and CEUS | Single-center study | Authors investigated the perfusion by CEUS as objective marker to distinguishing between the inflammation and fibrosis. | |
| Prospective | No correlation was found between the severity of inflammation or fibrosis on histopathology and the degree of enhancement (P =0.45 for inflammation and P =0.19 for fibrosis); Wall thickness assessed by US correlated with both, histological inflammation (P =0.001) and fibrosis (P =0.048). | |||
| Chen et al. (2018) [24] | USE (SWE) | Single-center study | The aim of this study was to investigated whether the quantification of stiffness using USE is able to distinguishing between the inflammation and fibrotic component of strictures. | |
| Prospective | A cutoff of 22.55 kPa can discriminate mild to moderate and severe fibrosis (sensitivity 69.6%, specificity 91.7% with AUC of 0.822; P =0.002). | |||
| Ding et al. (2019) [25] | USE (SWE, strain ratio, ARFI) | Single-center study | To evaluate the diagnostic performance of USE (different modalities) for assessment of the predominant types of intestinal stenosis. | |
| Prospective | The optimal cutoff value to discriminate predominantly fibrotic strictures on point-SWE was >2.73 m/s (sensitivity, 75%; specificity, 100%; accuracy, 96%; AUROC, 0.833; P<0.05). | |||
| Point-SWE outperforms ARFI and strain ratio for strictures characterization. | ||||
MRE, magnetic resonance enterography; DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient; DCE, dynamic contrast enhancement; MTR, magnetization transfer ratio; MT, magnetization transfer; AUC, area under received operating characteristic curve; USE, ultrasound elastography; US, ultrasound; SWE, shear wave elastography; CEUS, contrast-enhanced ultrasound; ARFI, acoustic radiation force impulse.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download