1. Duricova D, Burisch J, Jess T, Gower-Rousseau C, Lakatos PL; ECCO-EpiCom. Age-related differences in presentation and course of inflammatory bowel disease: an update on the population-based literature. J Crohns Colitis. 2014; 8:1351–1361.

2. Burisch J, Pedersen N, Čuković-Čavka S, et al. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014; 63:588–597.

3. Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012; 6:965–990.

4. Lakatos L, Kiss LS, David G, et al. Incidence, disease phenotype at diagnosis, and early disease course in inflammatory bowel diseases in Western Hungary, 2002-2006. Inflamm Bowel Dis. 2011; 17:2558–2565.

5. Loftus CG, Loftus EV Jr, Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007; 13:254–261.

6. Shivananda S, Lennard-Jones J, Logan R, et al. Incidence of in flammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut. 1996; 39:690–697.

7. Moum B, Vatn MH, Ekbom A, et al. Incidence of ulcerative colitis and indeterminate colitis in four counties of southeastern Norway, 1990-93: a prospective population-based study: the Inflammatory Bowel South-Eastern Norway (IBSEN) Study Group of Gastroenterologists. Scand J Gastroenterol. 1996; 31:362–366.

8. Hudson M, Flett G, Sinclair TS, Brunt PW, Templeton A, Mowat NA. Fertility and pregnancy in inflammatory bowel disease. Int J Gynaecol Obstet. 1997; 58:229–237.

9. Khosla R, Willoughby CP, Jewell DP. Crohn’s disease and pregnancy. Gut. 1984; 25:52–56.

10. Cornish JA, Tan E, Teare J, et al. The effect of restorative proctocolectomy on sexual function, urinary function, fertility, pregnancy and delivery: a systematic review. Dis Colon Rectum. 2007; 50:1128–1138.

11. Mountifield R, Bampton P, Prosser R, Muller K, Andrews JM. Fear and fertility in inflammatory bowel disease: a mismatch of perception and reality affects family planning decisions. Inflamm Bowel Dis. 2009; 15:720–725.

12. Marri SR, Ahn C, Buchman AL. Voluntary childlessness is increased in women with inflammatory bowel disease. Inflamm Bowel Dis. 2007; 13:591–599.

13. Johnson P, Richard C, Ravid A, et al. Female infertility after ileal pouch-anal anastomosis for ulcerative colitis. Dis Colon Rectum. 2004; 47:1119–1126.

14. Olsen KO, Joelsson M, Laurberg S, Oresland T. Fertility after ileal pouch-anal anastomosis in women with ulcerative colitis. Br J Surg. 1999; 86:493–495.

15. Seiler JS. Pelvic pathology affecting fertility. Int J Fertil. 1987; 32:208–212.
16. Oresland T, Palmblad S, Ellström M, Berndtsson I, Crona N, Hultén L. Gynaecological and sexual function related to anatomical changes in the female pelvis after restorative proctocolectomy. Int J Colorectal Dis. 1994; 9:77–81.

17. McHugh SM, Rifkin IR, Deighton J, et al. The immunosuppressive drug thalidomide induces T helper cell type 2 (Th2) and concomitantly inhibits Th1 cytokine production in mitogen- and antigen-stimulated human peripheral blood mononuclear cell cultures. Clin Exp Immunol. 1995; 99:160–167.

18. Arkuran C, McComb P. Crohn’s disease and tubal infertility: the effect of adhesion formation. Clin Exp Obstet Gynecol. 2000; 27:12–13.
19. Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod Update. 2001; 7:567–576.

20. Selinger CP, Eaden J, Selby W, et al. Patients’ knowledge of pregnancy-related issues in inflammatory bowel disease and validation of a novel assessment tool (‘CCPKnow’). Aliment Pharmacol Ther. 2012; 36:57–63.

21. Maheshwari A, Fowler P, Bhattacharya S. Assessment of ovarian reserve: should we perform tests of ovarian reserve routinely? Hum Reprod. 2006; 21:2729–2735.

22. van Rooij IA, Broekmans FJ, Hunault CC, et al. Use of ovarian reserve tests for the prediction of ongoing pregnancy in couples with unexplained or mild male infertility. Reprod Biomed Online. 2006; 12:182–190.

23. Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006; 12:685–718.

24. La Marca A, Giulini S, Tirelli A, et al. Anti-Müllerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod. 2007; 22:766–771.

25. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006; 21:3103–3107.

26. Dayal M, Sagar S, Chaurasia A, Singh U. Anti-Mullerian hormone: a new marker of ovarian function. J Obstet Gynaecol India. 2014; 64:130–133.

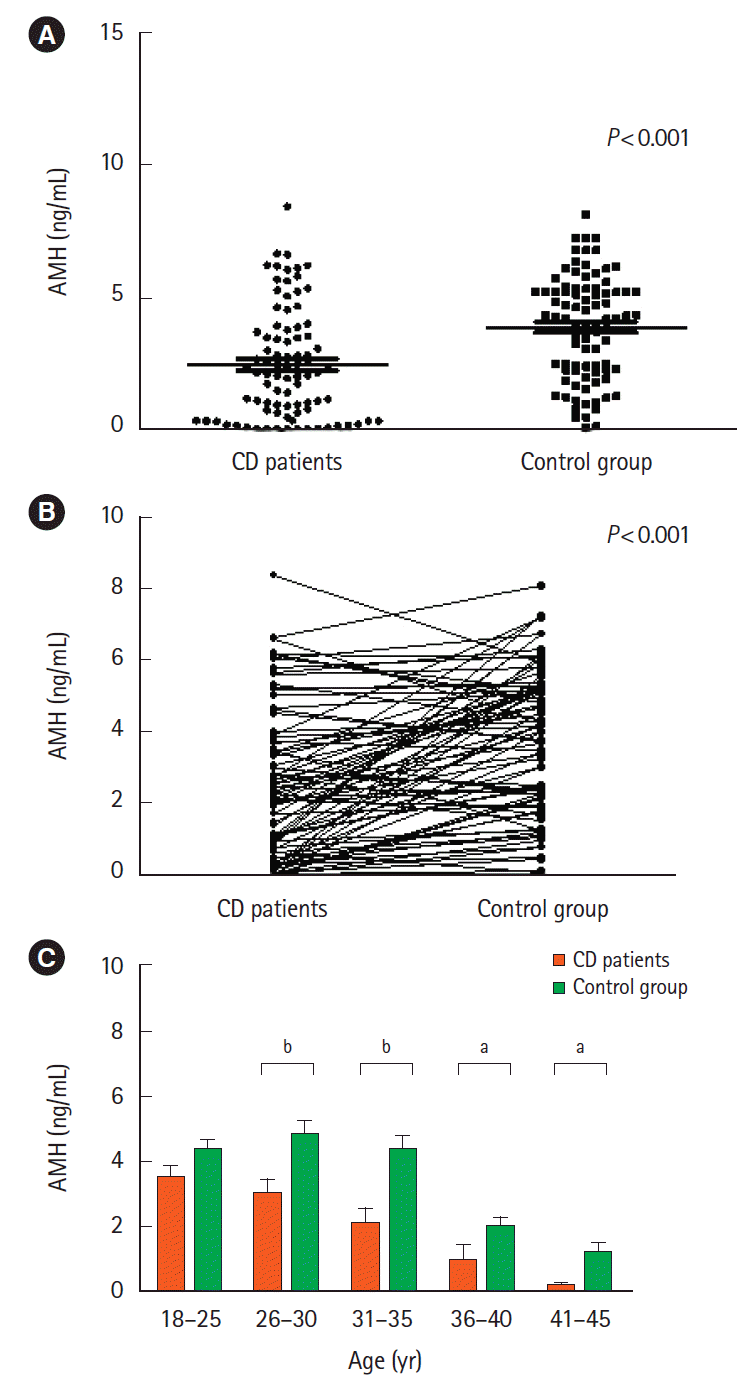
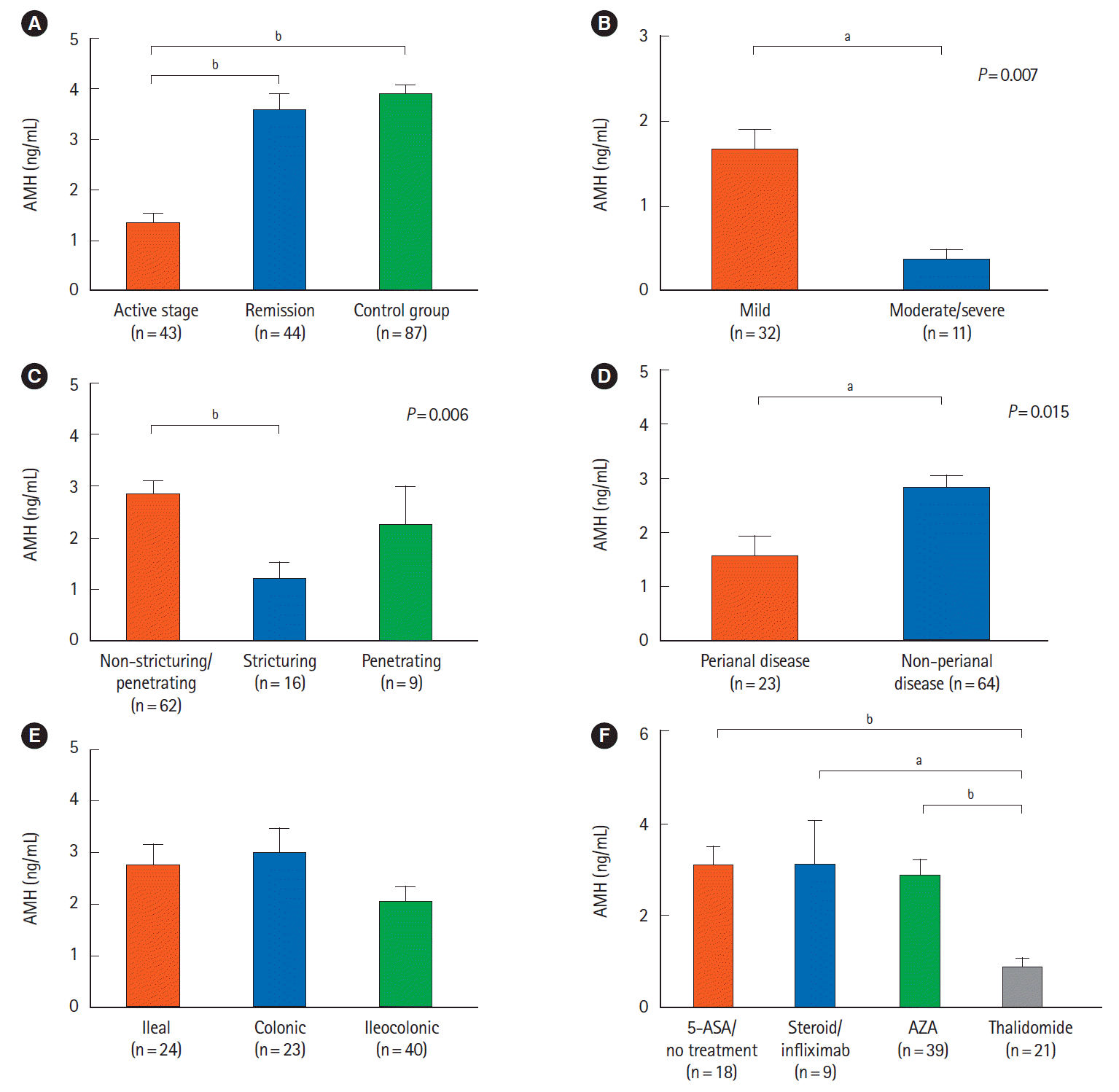
27. Fréour T, Miossec C, Bach-Ngohou K, et al. Ovarian reserve in young women of reproductive age with Crohn’s disease. Inflamm Bowel Dis. 2012; 18:1515–1522.

28. Şenateş E, Çolak Y, Erdem ED, et al. Serum anti-Müllerian hormone levels are lower in reproductive-age women with Crohn’s disease compared to healthy control women. J Crohns Colitis. 2013; 7:e29–e34.

29. Peng X, Zhi M, Wei M, et al. Thalidomide results in diminished ovarian reserve in reproductive age female IBD patients. Medicine (Baltimore). 2017; 96:e6540.

30. La Marca A, Sighinolfi G, Giulini S, et al. Normal serum concentrations of anti-Müllerian hormone in women with regular menstrual cycles. Reprod Biomed Online. 2010; 21:463–469.

31. Ferraretti AP, La Marca A, Fauser BC, et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011; 26:1616–1624.

32. Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology. 2016; 150:1393–1407.

33. Chow SC, Shao J, Wang H. Sample size calculations in clinical research. Boca Raton: Chapman & Hall/CRC Biostatistics Series,;2008.
34. van Rooij IA, Broekmans FJ, te Velde ER, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002; 17:3065–3071.
35. Iwase A, Nakamura T, Nakahara T, Goto M, Kikkawa F. Anti-Müllerian hormone and assessment of ovarian reserve after ovarian toxic treatment: a systematic narrative review. Reprod Sci. 2015; 22:519–526.

36. Browne H, Armstrong A, Decherney A, et al. Assessment of ovarian function with anti-Müllerian hormone in systemic lupus erythematosus patients undergoing hematopoietic stem cell transplant. Fertil Steril. 2009; 91(4 Suppl):1529–1532.

37. Henes M, Froeschlin J, Taran FA, et al. Ovarian reserve alterations in premenopausal women with chronic inflammatory rheumatic diseases: impact of rheumatoid arthritis, Behçet’s disease and spondyloarthritis on anti-Müllerian hormone levels. Rheumatology (Oxford). 2015; 54:1709–1712.

38. Chen M, Shen B. Comparable short- and long-term outcomes of colonoscopic balloon dilation of Crohn’s disease and benign non-Crohn’s disease strictures. Inflamm Bowel Dis. 2014; 20:1739–1746.

39. Veloso FT. Clinical predictors of Crohn’s disease course. Eur J Gastroenterol Hepatol. 2016; 28:1122–1125.

40. Bharadwaj S, Fleshner P, Shen B. Therapeutic armamentarium for stricturing Crohn’s disease: medical versus endoscopic versus surgical approaches. Inflamm Bowel Dis. 2015; 21:2194–2213.
41. Lazzerini M, Bramuzzo M, Martelossi S, Magazzù G, Pellegrino S, Ventura A. Amenorrhea in women treated with thalidomide: report of two cases and literature review. Inflamm Bowel Dis. 2013; 19:E10–E11.
42. Song T, Ma X, Gu K, et al. Thalidomide represses inflammatory response and reduces radiculopathic pain by inhibiting IRAK-1 and NF-κB/p38/JNK signaling. J Neuroimmunol. 2016; 290:1–8.

43. Amirshahrokhi K, Khalili AR. The effect of thalidomide on ethanol-induced gastric mucosal damage in mice: involvement of inflammatory cytokines and nitric oxide. Chem Biol Interact. 2015; 225:63–69.

44. Amirshahrokhi K. Anti-inflammatory effect of thalidomide in paraquat-induced pulmonary injury in mice. Int Immunopharmacol. 2013; 17:210–215.

45. Sharma GT, Dubey PK, Kumar GS. Effects of IGF-1, TGF-alpha plus TGF-beta1 and bFGF on in vitro survival, growth and apoptosis in FSH-stimulated buffalo (Bubalis bubalus) preantral follicles. Growth Horm IGF Res. 2010; 20:319–325.

46. Bramuzzo M, Ventura A, Martelossi S, Lazzerini M. Thalidomide for inflammatory bowel disease: systematic review. Medicine (Baltimore). 2016; 95:e4239.
47. He Y, Mao R, Chen F, et al. Thalidomide induces clinical remission and mucosal healing in adults with active Crohn’s disease: a prospective open-label study. Therap Adv Gastroenterol. 2017; 10:397–406.

48. Zhu Z, Li M, Shu X, et al. Thalidomide is a therapeutic agent that is effective in inducing and maintaining endoscopic remission in adult CD patients. Clin Res Hepatol Gastroenterol. 2017; 41:210–216.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download