Abstract
Although ulcerative colitis (UC) is confined to colonic and rectal mucosa in a continuous fashion, recent studies have also demonstrated the involvement of upper gastrointestinal tract as diagnostic endoscopy becomes more available and technically advanced. The pathogenesis of UC is not well established yet. It might be associated with an inappropriate response of host mucosal immune system to gut microflora. Although continuous and symmetric distribution of mucosal inflammation from rectum to colon is a typical pattern of UC, clinical feature and course of atypically distributed lesions in UC might also help us understand the pathogenesis of UC. Herein, we report a case of duodenal involvement of UC which successfully remitted after infliximab therapy. Endoscopic and pathologic findings before and after administration of anti-tumor necrosis factor suggest that the pathogenesis of upper gastrointestinal involvement of UC may be similar to that of colon involvement.
Ulcerative colitis (UC) is characterized by chronic mucosal inflammation and confined to rectum and colon unlike CD which can involve whole GI tract from esophagus to anus. Recently, some studies have demonstrated the involvement of upper GI tract in patients with UC as diagnostic endoscopy becomes more available and technically advanced. However, its clinical course and the association with colonic lesion are still unknown because it is very rare. Here, we report a patient with acute exacerbated UC and symptomatic diffuse duodenitis which was successfully treated with infliximab.
A 45-year-old male who had a family history of UC visited Daehang Hospital presenting with abdominal pain and frequent (>10/day) bloody diarrhea. He was diagnosed with left-sided UC about 10 years ago. He maintained remission with combination mesalamine therapy. The patient was admitted to our hospital and intravenous corticosteroid (hydrocortisone 300 mg/day) was started. On admission, his body temperature was 38.8°C. Blood test revealed elevation of CRP, leukocytosis and mild anemia as follows; CRP 4.0 mg/dL, white blood cells 12,900/μL, and hemoglobin 10.4 g/dL. Colonoscopy showed diffuse and ulcerative inflammation with spontaneous mucosal hemorrhage and profuse mucopurulent exudates from the rectum to descending colon in a continuous and symmetric fashion (Fig. 1A).
For a week, bloody diarrhea persisted despite intravenous infusion of corticosteroid. The patient also complained of severe epigastric pain and vomiting. We added proton pump inhibitor, but his symptoms did not improve. Simple abdominal radiography was performed and it showed no sign of intestinal obstruction or toxin megacolon. We highly recommended esophagogastroduodenoscopy (EGD) which showed diffuse edematous and ulcerative inflammation on the bulb and 2nd portion of duodenum (Fig. 1C). On histopathologic examination, marked inflammatory cell infiltration and cryptitis were noted without evidence of granuloma or inclusion body (intranuclear or intracytoplasmic) (Fig. 2A). Helicobacter pylori was not detected. We started standard induction therapy of infliximab (300 mg infusion at 0, 2nd and 6th weeks). His epigastric symptom and bloody diarrhea improved abruptly. Three months later, follow-up colonoscopy and EGD showed mucosal healing with whitish scarring (Fig. 1B and D). On histopathologic examination of duodenal mucosa, there was decreased density of inflammatory cell infiltrates in lamina propria with decreased active inflammation compared to those at prior medical treatment. Instead of prominent inflammatory cell infiltrates, subepithelial fibrosis was noted (Fig. 2B). After more than 1 year, the patient is still sustaining clinical remission with infliximab maintenance therapy.
UC is an idiopathic IBD characterized by mucosal inflammation in a continuous fashion from rectum to colon. However, recent studies have reported that colonic skip lesions such as patchy or segmental inflammation can be found in patients with UC as well as CD [1,2]. Another exception of conventional definition of UC can also be encountered in practice. Some case reports have demonstrated gastroduodenal or enteric involvement of UC along with advancement of endoscopic imaging techniques [3-6]. Thompson and Bargen [6] firstly reported 2 cases of ulcerative duodenitis in patients with UC in 1960. Since then, some case reports or cases series have been published (Table 1) [7-16]. In 1990s, Ruuska et al. [3] prospectively evaluated upper GI involvement of 47 pediatric UC patients (esophageal ulcer in 2, gastritis in 22, duodenitis or duodenal ulcer in 18) and designated them simply as “nonspecific inflammation.” Japanese reports suggested that the prevalence of gastroduodenal involvement of UC was 4.7% to 7.6%. Such involvement of UC was only found in cases with pancolitis or in cases with proctocolectomy [11,13].
Symptoms of gastroduodenal involvement may vary, including epigastric pain, nausea and vomiting. Asymptomatic or screening cases are more common, accounting for 60% to 75% of cases [11,13]. In our case, the patient had severe epigastric pain, which was different from the usual pattern or location of UC. The symptom did not improve despite intravenous injection of proton pump inhibitor. After induction therapy with infliximab, his epigastric symptom improved abruptly before clinical improvement of UC symptoms such as diarrhea and hematochezia. Histopathologic characteristics of duodenitis of UC is similar to UC, showing neutrophilic cryptitis, crypt abscess and basal lymphoplasmacytosis without pathognomonic finding. Therefore, other chronic conditions such as Helicobacter-related gastroduodenitis, CD, lymphoma and opportunistic viral infections need to be ruled out for differential diagnosis.
Standard therapy for gastroduodenitis of UC has not been established yet. However, for a symptomatic case, 5-aminosalicylate, corticosteroid and anti-TNF can be used, similar to treatment for colitis of UC. In literature review, 3 cases of diffuse duodenitis of UC have been successfully treated with anti-TNF (Table 1) [12,15,16]. All of them were proctocolectomized cases, so only gastroduodenal mucosa was evaluated by EGD. However, in our case, we could compare endoscopic and microscopic findings of both colon and duodenal inflammation before and after infliximab therapy (Figs. 1 and 2). Follow-up EGD and colonoscopy demonstrated simultaneous endoscopic and pathologic remission of both duodenal and colonic mucosal inflammation as well as clinical remission. This might be associated with common pathogenic process in upper GI inflammation of UC, although the pathophysiology of UC is not well-known yet. In the case of upper gastroduodenal involvement of UC without symptom, it is currently unclear whether it might be related to poor prognosis like CD or it may require medical therapy targeting upper GI inflammation. Given the fact that most cases with upper GI involvement of UC had pancolitis or underwent proctocolectomy, it might be associated with extensive or severe behavior of UC. Similarly, the clinical significance of periappendiceal orifice inflammation or right-sided skip inflammation of UC is controversial [1,2,17,18]. Long-term clinical course of UC with upper GI tract as well as colonic skip inflammation should be evaluated to elucidate the pathophysiology of UC.
In conclusion, here we report a patient with acute exacerbated UC and symptomatic diffuse duodenitis successfully treated with infliximab. This case demonstrates that endoscopic and pathologic remission of mucosal inflammation of upper GI tract and colonic lesion of UC can be achieved by infliximab. This suggests that the pathogenesis of upper GI involvement of UC may be similar to that of colon involvement. We believe that this case can help us understand various behaviors of UC and predict its clinical course.
Notes
REFERENCES
1. Mutinga ML, Odze RD, Wang HH, Hornick JL, Farraye FA. The clinical significance of right-sided colonic inflammation in patients with left-sided chronic ulcerative colitis. Inflamm Bowel Dis. 2004; 10:215–219.

2. Choi YS, Kim WJ, Kim JK, Kim DS, Lee DH. Efficacy of topical 5-aminosalicylate monotherapy in patients with ulcerative proctitis with skip inflammation. J Gastroenterol Hepatol. 2018; 33:1200–1206.

3. Ruuska T, Vaajalahti P, Arajärvi P, Mäki M. Prospective evaluation of upper gastrointestinal mucosal lesions in children with ulcerative colitis and Crohn’s disease. J Pediatr Gastroenterol Nutr. 1994; 19:181–186.

4. Kaufman SS, Vanderhoof JA, Young R, Perry D, Raynor SC, Mack DR. Gastroenteric inflammation in children with ulcerative colitis. Am J Gastroenterol. 1997; 92:1209–1212.
5. Mitomi H, Atari E, Uesugi H, et al. Distinctive diffuse duodenitis associated with ulcerative colitis. Dig Dis Sci. 1997; 42:684–693.
6. Thompson JW 3rd, Bargen JA. Ulcerative duodenitis and chronic ulcerative colitis: report of two cases. Gastroenterology. 1960; 38:452–455.

7. Valdez R, Appelman HD, Bronner MP, Greenson JK. Diffuse duodenitis associated with ulcerative colitis. Am J Surg Pathol. 2000; 24:1407–1413.

8. Terashima S, Hoshino Y, Kanzaki N, Kogure M, Gotoh M. Ulcerative duodenitis accompanying ulcerative colitis. J Clin Gastroenterol. 2001; 32:172–175.

9. Rubenstein J, Sherif A, Appelman H, Chey WD. Ulcerative colitis associated enteritis: is ulcerative colitis always confined to the colon? J Clin Gastroenterol. 2004; 38:46–51.
10. Kawai K, Watanabe T, Nakayama H, Roberts-Thomson I, Nagawa H. Images of interest. Gastrointestinal: small bowel inflammation and ulcerative colitis. J Gastroenterol Hepatol. 2005; 20:1791.

11. Hori K, Ikeuchi H, Nakano H, et al. Gastroduodenitis associated with ulcerative colitis. J Gastroenterol. 2008; 43:193–201.

12. Akitake R, Nakase H, Tamaoki M, Ueno S, Mikami S, Chiba T. Modulation of Th1/Th2 balance by infliximab rescues postoperative occurrence of small-intestinal inflammation associated with ulcerative colitis. Dig Dis Sci. 2010; 55:1781–1784.

13. Hisabe T, Matsui T, Miyaoka M, et al. Diagnosis and clinical course of ulcerative gastroduodenal lesion associated with ulcerative colitis: possible relationship with pouchitis. Dig Endosc. 2010; 22:268–274.

14. Lin J, McKenna BJ, Appelman HD. Morphologic findings in upper gastrointestinal biopsies of patients with ulcerative colitis: a controlled study. Am J Surg Pathol. 2010; 34:1672–1677.

15. Chiba M, Ono I, Wakamatsu H, Wada I, Suzuki K. Diffuse gastroduodenitis associated with ulcerative colitis: treatment by infliximab. Dig Endosc. 2013; 25:622–625.

16. Willington AJ, Taylor G, White J, Gearry RB. Gastrointestinal: ulcerative colitis-associated duodenitis. J Gastroenterol Hepatol. 2018; 33:973.

17. Byeon JS, Yang SK, Myung SJ, et al. Clinical course of distal ulcerative colitis in relation to appendiceal orifice inflammation status. Inflamm Bowel Dis. 2005; 11:366–371.

18. Naves JE, Lorenzo-Zúñiga V, Marín L, et al. Long-term outcome of patients with distal ulcerative colitis and inflammation of the appendiceal orifice. J Gastrointestin Liver Dis. 2011; 20:355–358.
Fig. 1.
Endoscopic findings. (A) At initial colonoscopy, diffuse ulcerative inflammation with profuse exudation and spontaneous mucosal hemorrhage. (B) At 3 months follow-up colonoscopy after induction therapy with infliximab, mucosal healing showing whitish scar formation was noted. (C) At initial esophagogastroduodenoscopy (EGD), diffuse edematous and ulcerative inflammation on the bulb and 2nd portion of duodenum. (D) At 3 months follow-up EGD after infliximab induction therapy, endoscopic mucosal healing was achieved on the duodenal mucosa showing scar change.
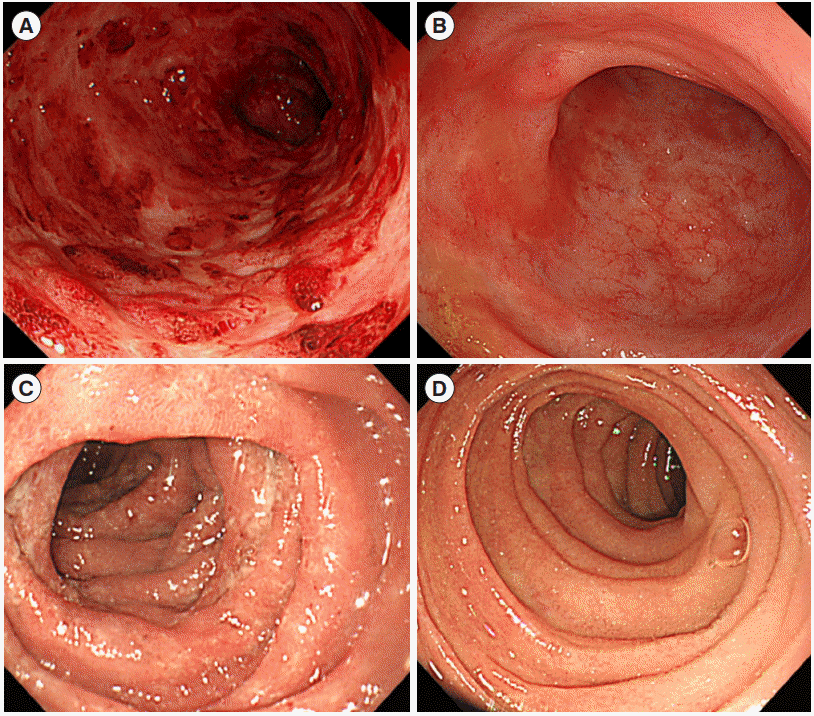
Fig. 2.
Histopathological findings. (A) High-power magnification of duodenum showing histologic features of chronic active duodenitis. There is a manifestation of chronic active colitis with crypt distortion, basal lymphoplasmacytosis and crypt abscess (H&E stain, ×200). (B) High-power magnification of duodenum after infliximab treatment. Note the decreased density of inflammatory cell infiltrates in lamina propria as well as decreased active inflammation compared to those of prior medical treatment. Instead of prominent inflammatory cell infiltrates, subepithelial fibrosis is also noted (H&E stain, ×200).
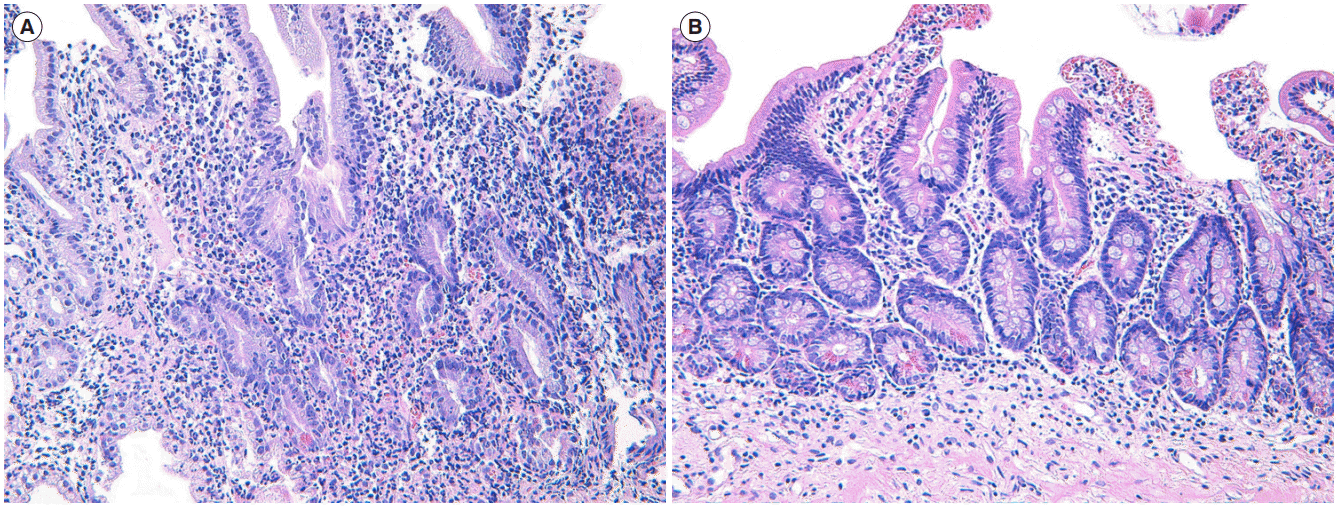
Table 1.
Review of the Literatures Concerning Upper Duodenal Involvement of UC (from 2000 to Present)
| Author (year) | Sex/age (yr) | UGI symptom | Extent of UC | History of proctocolectomy | Endoscopic features | Histologic features | Treatment | Other comments |
|---|---|---|---|---|---|---|---|---|
| Valdez et al. (2000) [7] | F/20 | Colitis symptom | E3 | (+) Subtotal colectomy | Not described | UC-like active duodenitis | Corticosteroid, sulfasalazine, metronidazole | Backwash ileitis (+) |
| F/62 | Recurrent nausea, vomiting, diarrhea | E3 | (+) Total colectomy | Not described | UC-like active duodenitis | Corticosteroid, azathioprine, cyclosporine | Backwash ileitis (–) | |
| M/17 | Excessive ileostomy output | E3 | (+) Subtotal colectomy | Not described | UC-like active duodenitis | Corticosteroid | Backwash ileitis (+) | |
| Terashima et al. (2001) [8] | M/31 | Nausea, epigastralgia | E3 | (–) Subsequently, subtotal colectomy | Stenosis by mucosal edema with multiple erosions and granular change | Severe lympho- plasmacytosis with crypt abscess | Corticosteroid | Backwash ileitis (–) |
| F/30 | Nausea, epigastralgia, hematochezia | E2 | (+) Subtotal proctocolectomy | Multiple erosions and ulcers, pseudopolyposis | Severe lympho-plasmacytosis and cryptitis | Corticosteroid | Backwash ileitis (–) | |
| Rubenstein et al. (2004) [9] | M/38 | Abdominal pain, non-bloody emesis, weight loss | E3 | (+) Near total proctocolectomy | Diffusely ulcerated mucosa with patchy white exudates | Crypt distortion and plasmacytosis | Corticosteroid, azathioprine | Family history of UC (+), backwash ileitis (–) |
| Kawai et al. (2005) [10] | M/- | Persistent epigastric discomfort | - | (+) Total colectomy | Diffuse ulceration | Severe lympho-plasmacytosis | 5-ASA | - |
| Akitake et al. (2010) [12] | F/19 | Vomiting and epigastralgia | E3 | (+) Proctocolectomy | Edema and granularity | Cryptitis | Infliximab | Backwash ileitis (–) |
| Chiba et al. (2013) [15] | M/58 | Tarry stool | - | (+) Subtotal colectomy | Diffuse friable mucosa with ulcer & luminal narrowing | Marked inflammation cell infiltration with cryptitis | Infliximab | - |
| Willington et al. (2018) [16] | F/57 | Epigastric pain | - | (+) Panproctocolectomy | Severe duodenitis | Ulceration with neutrophil and plasmocytosis | Adalimumab → infliximab | - |
| Choi et al. (current study) | M/45 | Vomiting, epigastric pain | E2 | (-) Colectomy | Diffuse edematous and ulcerative inflammation | Marked inflammatory cell infiltration and cryptitis | Infliximab | Family history of UC (+), backwash ileitis (–) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download