Abstract
Background/Aims
The onset of inflammatory bowel disease (IBD) usually occurs at young age, and therefore, women IBD patients experience pregnancy during their disease progression. Recently, the use of anti-tumor necrosis factor-α (anti-TNF-α) has been rapidly increasing. The aim of this study was to evaluate pregnancy related outcomes in women with IBD who were treated with anti-TNF-α during pregnancy and immunity of their children.
Methods
Korean women with IBD who had been treated with anti-TNF-α during pregnancy had been enrolled. Medical records were reviewed and a survey was performed for each patient. For the patients who agreed on additional examination for their children, children’s growth, medical history and antibody to hepatitis B surface antigen (anti-HBs) titer were checked.
Results
All 18 patients had been diagnosed with Crohn’s disease. There was not any case of preterm delivery, low birth-weight infant, congenital anomaly, nor stillbirth. All 12 children had followed the regular vaccination schedule for hepatitis B and 4 of them showed negative results for anti-HBs. After the 1 booster vaccination, all children demonstrated seroconversion. Regarding live vaccines, 4 children had bacillus Calmette-Guerin and 4 had rotavirus vaccine before 6 months, without any specific side effects.
Conclusions
This was the first study of immunity of the children born from IBD women who had been treated with anti-TNF-α medication during their pregnancy. IBD women had comparable pregnancy outcomes with the general women population, suggesting that the disease activity rather than the administered medication would be more important in healthy pregnancy. Considering the history of vaccination and anti-HBs titers, immunity seems to be intact in the children.
Go to : 
Inflammatory bowel disease (IBD) commonly develops during young age and this period belongs to the reproductive age [1-3]. Especially, women IBD patients would experience pregnancy and delivery along with the disease. In addition, as IBD is a chronic disease and patients should receive long-term medical therapies, IBD women would also have to continue medication even during their pregnancies.
In previous studies, pregnant IBD women’s disease activity seemed to affect the pregnancy-related outcomes such as preterm delivery, miscarriage and normal delivery rate [4-8]. For example, if pregnant IBD women maintained the remission state of the disease, perinatal complications and normal delivery rate were similar with healthy subjects [4-7,9,10]. However, if pregnant IBD women’s clinical status were active, the rate of spontaneous abortion and preterm delivery was higher than healthy subjects and their disease itself also seemed to worsen [4-8,11]. Therefore, the continuation of maintenance therapy seems necessary for reproductive-aged women to maintain remission state, and also, additional medical treatment seems inevitable when the disease activity becomes worse [8,12-17]. As a result, recently updated treatment protocols have recommended the recognition of the influence of medication to pregnant IBD women and the use of safe medication with appropriate duration to maintain the remission state [8].
Recently, anti-TNF-α agents have shown effectiveness in cases of refractory or severe IBD and its use is rapidly increasing [18,19]. These drugs consist of IgG1 monoclonal antibody (Ab) for TNF-α, and infliximab and adalimumab are the most commonly used. The Food and Drug Administration (FDA) divides drugs into categories A, B, C, D, and X in pregnancy. U.S. and European guidelines classifies infliximab and adalimumab as category B (no evidence of risk in humans) [8,20]. However, Australian Drug Evaluation Committee (ADEC) pregnancy category is different from FDA categories. Australia classifies them as category C (Drugs that have caused, or may be suspected of causing, harmful effects on the human fetus or neonate without causing malformations. These effects may be reversible) [21]. Anti-TNF-α agents may cross the placenta after 20 weeks gestational age, and can remain in the blood of infants until 3 to 6 months and in maximum up to 1 year after the last administration [22-24]. In the United States and Europe, the 2011 London Position Statement on Biological Therapy for IBD describes that the risk of pregnancy and breastfeeding of mother and fetus for anti-TNF-α therapy is due to active IBD, but not medication, and that the use in the first 2 trimesters is low risk [8]. Many studies on pregnant IBD women treated with anti-TNF-α did not show significant increase of perinatal complication. However, in the 2 largest safety studies on the infliximab and pregnancy in the United States, the TREAT registry and the Infliximab Safety Database, some fetal malformations and perinatal complications such as preterm births and stillbirth have been reported [5,25]. In addition, its influence on their children’s future growth also remain inconclusive [25-33].
Therefore, this study aimed to evaluate the pregnancy-related outcomes in IBD women who had received anti-TNF-α therapy during pregnancy, and the immunity of their children such as serologic responses to vaccine and serious infection rate.
Go to : 
This study was a multicenter, retrospective and cross-sectional study. IBD women with history of delivery or currently pregnant from January 2009 to May 2014 were included, from 3 tertiary hospitals in Korea. The inclusion criteria were 19 to 45 years old women having diagnosed as IBD according to clinical history, endoscopic, histologic, and radiologic examinations, having experienced delivery or pregnancy after IBD diagnosis, and having been treated with anti-TNF-α during pregnancy. Patients with history of specific disease or drug history which may affect pregnancy, BMI under 18.5 kg/m2, and several medical conditions such as severe heart or lung disease, uncontrolled end stage renal disease, uncompensated liver cirrhosis, and history of malignancy were excluded from the study.
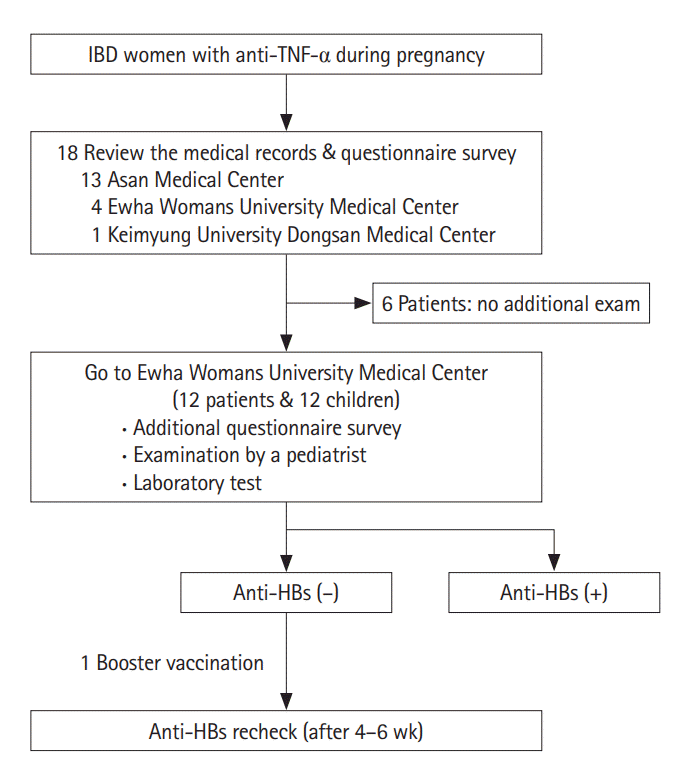
Personal medical records were reviewed and a questionnaire survey was performed for each patient. Baseline clinical characteristics, history of any other disease and medication, time, number, and cessation of anti-TNF-α therapy during pregnancy were extracted from the medical records. The questionnaire survey evaluated obstetrical history, delivery methods, gestational age at delivery, pregnancy outcomes (preterm delivery, low birth-weight infant, congenital anomaly, and stillbirth), adverse effects related with medication, and improvement and aggravation of the disease during pregnancy. The history of vaccination was evaluated by vaccination record card for each child, and body weight at birth and disease history were collected from mother’s survey (Fig. 1).
To conduct immunological studies on children of IBD women who received anti-TNF-α treatment during pregnancy, children with complete 3 regular vaccinations (0, 1, 6 months) of HBV were included. For the patients who agreed on additional examination for their children, pediatric doctor’s examination and laboratory tests were performed. Pediatric doctors performed general physical examinations and checked the growth and development of each child. Date of birth, birth weight, perinatal complication, vaccination history and medical history were acquired from their parents and laboratory tests (hemoglobin, AST, ALT, and anti-HBs) were also performed. The anti-HBs titer was measured by commercial enzyme immunoassay, and the result was considered negative when it was under 10 international units per liter. For the children whose anti-HBs titer was negative, booster vaccination was performed by intramuscular injection using 10 μg of purified HBs protein (0.5 mL; Hepavax-Gene® TF, Janssen, Incheon, South Korea). It was interpreted as positive immunogenetic response and intact immunological memory if the child’s Ab was over 10 units per liter after 4 to 6 weeks (Fig. 1).
This study was approved by the Institutional Review Board of Ewha Womans University Mokdong Hospital (IRB No. 2014-06-004). Written informed consent for the use of clinical records and performing survey was provided by all participants.
Go to : 
Eighteen women who had experienced delivery after the IBD diagnosis were enrolled in this study. All 18 subjects had been diagnosed with CD without any other specific underlying diseases. Mean age at delivery was 31.9 ± 3.3 years (range, 25–38 years) and mean disease duration until delivery was 9.8±3.9 years (range, 2.8–18.0 years). The operation history related with IBD was observed in 7 patients and experience of alcohol or smoking during pregnancy was not observed in any patient. Combination therapy with immunomodulatory during pregnancy was observed in 3 patients. One patient had used adalimumab with azathioprine, and 2 patients who had experienced abortion during the previous pregnancy had used combination therapy of infliximab and azathioprine. The last anti-TNF-α administration was performed between 22 and 32 weeks of gestational age and 4 patients maintained their treatment until second trimester between 22 to 27 weeks (Table 1).
Baseline Clinical Characteristics of IBD Women Treated with Anti-TNF-α during Pregnancy (n=18)
All 18 patients did not discontinue the medication by themselves throughout the pregnancy. There was no aggravation of symptoms during pregnancy and nor anti-TNF-α related adverse effects. Mean gestational weeks at delivery was 38.8 ± 1.2 weeks (range, 37–41 weeks) and mean birth weight of infants was 2.95±0.30 kg (range, 2.50–3.59 kg). Normal delivery was performed in 4 patients and cesarean section was performed in 12 patients. There was no maternal complication related to pregnancy and delivery. There was no preterm delivery, low birth-weight infant, congenital anomaly, nor stillbirth.
Among 18 patients, 12 agreed with additional examination for their children. There were 4 boys and 8 girls, their mean age at examination was 28.3±16.6 months and age range was between 8 to 54 months. All children showed normal development. Percentile of body weight was 42.6 ± 28.5 (range, 3–92 percentile), and 2 children (16.7%) was below the 10 percentile range.
All 12 children had followed the regular vaccination schedule for hepatitis B (0, 1, 6 month) and 4 (33.3%) were negative for anti-HBs. Four to six weeks after the 1 booster vaccination, all children (100%) demonstrated seroconversion. Admission history due to infections before 12 months of age was found from 3 children whose anti-HBs result was negative. Their diagnoses of hospitalization were bronchitis, colitis, and pneumonia, and they were discharged without having any specific complications.
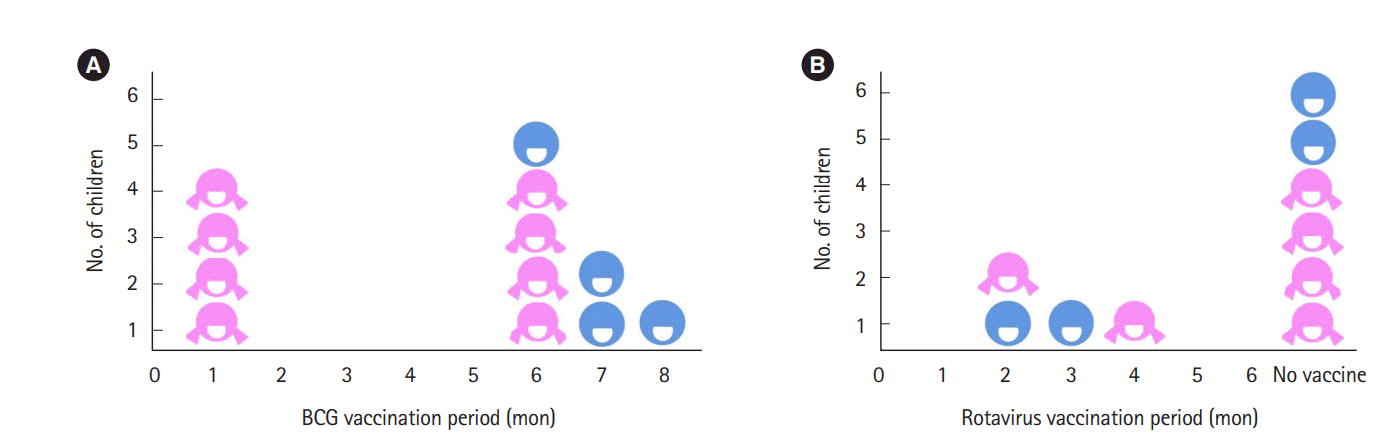
Reviewing the live vaccines administered to the children, 4 children had BCG at 1 month and 4 had rotavirus vaccine before 4 months, with no specific adverse effects (Fig. 2). Total 7 children had taken live vaccination before 6 months, and they did not experience any complication.
Go to : 
Among the 18 female IBD patients who had delivered their child while receiving anti-TNF-α treatments, all subjects-maintained remission state without any significant complication including perinatal complications. Their average delivery age was 31.9 ± 3.3 years old, which was similar with Korean women population (32.6 years old). However, the average birth weight of infants born from IBD women was 2.95 ± 0.30 kg, which was lower than 3.2 kg of the Korean newborn population [34]. The method of delivery was vaginal delivery in 4 patients and cesarean section in 12 patients (75%), which was higher than Korean women population (42.6%) [34].
Past studies have shown conflicting results on safety of anti-TNF-α therapy to IBD women during pregnancy. The PIANO study performed in the United States had shown that the use of anti-TNF-α agents was not associated with any increase of the complications even when adjusted for disease type or disease activity of IBD [32]. Similarly, data from the TREAT registry also have shown that the rates of miscarriage and neonatal complications were not significantly different between infliximab-treated and infliximab-naive IBD patients [25]. In contrast, U.S. FDA database had demonstrated 61 anomalies, such as vertebral and cardiac anomalies in IBD mother’s children, who had been exposed to anti-TNF-α in utero [29]. There were several studies which demonstrated that disease activity when patients become pregnant affect the pregnancy-related outcomes and normal delivery rate [4-7]. In this study, all subjects maintained remission state during pregnancy continuing the medical treatment, and showed no perinatal complication. Therefore, even though the disease duration is long, pregnancy outcome would not be affected by the disease if patients could maintain the remission state during pregnancy.
No significant evidences of developmental problems were found in the children. Their average percentile weight when they had pediatric examination was 42.6% ± 28.5% which was similar to average Korean age-matched children. Approximately 16.7% of the children’s weight were under the 10 percentile range, which was slightly higher than the average, but the development of all children were within the normal range.
The positive rate of anti-HBs titer from IBD mother’s children who had taken regular hepatitis B vaccination was 66.7%, which was similar to 62.7%–78.6% resulted in general Korean age-matched children. Seroconversion rate after 1 booster vaccination was 100%, and this was also not lower than the general population, which was 88.8% [35]. Therefore, immunological memory for hepatitis B vaccination of these children seemed intact. Recently, the PIANO study have reported that adequate response to both tetanus and Haemophilus influenzae type b (Hib) vaccines were found in 92% infants [36], and another study performed in year 2017 had demonstrated that the Ab titer for Hib and tetanus toxin in children over 7 months old did not show significant difference between the biologic agent exposed group and the unexposed group during pregnancy [37]. However, there was a significant increase in infant infections from 9 to 11 months of age in the combination therapy group relative to the unexposed group [32]. In this study, 3 children whose anti-HBs were negative were admitted due to infections before 12 months of age, but they were discharged without any specific complication. It is reported that the drugs are detected in the blood of the children until 6 months after delivery, up to maximum of 12 months, when anti-TNF-α has been used during pregnancy. During that period, the possibility of infection may be high, and it can be assumed that antibodies were not produced sufficiently in children with weak immunity. However, they were well recovered without significant complications, and immunologic memory was intact after hepatitis B booster vaccination. Therefore, the correlation between anti-TNF-α during pregnancy and immunity in children is not clear. Since the number of children in this study was small, further research on more subjects is necessary to estimate their correlation.
Previous studies have demonstrated that if patients had undergone anti-TNF-α therapy during pregnancy, the monoclonal Ab may cross the placenta and be delivered to the fetus, and it can remain until 6 months, up to 12 months after birth [23,26-28,33]. However, there are also growing evidences demonstrating that following the anti-TNF-α medication even until the third trimester does not affect the fetus significantly [8]. In this study, there was no significant difference between the patients who had their last anti-TNF-α medication at the second trimester and the third trimester regarding their pregnancy outcomes and immunity of the children. Therefore, it would be more beneficial to continue anti-TNF-α treatment until 32 weeks of gestational age in the third trimester to prevent aggravation of disease activity rather than choosing the early discontinuation.
In this study, 7 children have had live vaccination, BCG and/or rotavirus, before 6 months old, but there were no significant side effects. Current guidelines suggest that children born from IBD patients who had been treated with anti-TNF-α medication during pregnancy can take inactive vaccination according to the regular vaccination schedule, because there are no evidences that intra-uterine exposure to TNF-α monoclonal Ab caused inactivated vaccine related side effects. However, it has been suggested that live vaccination should be performed after 6 months old or when the drug which had been treated to mother is not detected from the children’s blood [8]. However, studies on whether this is sufficient for disease prevention or its consequence on immunity acquisition with these live vaccination schedule are rare [17,31,38]. One study showed that there was no significant perinatal complication in 15 children from patients who received anti-TNF-α therapy during pregnancy who had BCG vaccination before 6 months old in the previous study [39]. Recent data show that the IgG, IgA levels were adequate, but IgM level was low in 50% infants who did not show higher infection rate [36]. However, one study showed that of the 40 children born from mothers who had biologic therapy, 7 of them had mild reaction after rotavirus vaccination [37]. In addition, there was a case report of a child who had disseminated BCG infection after BCG vaccination at 3 months old [40]. Therefore, further additional tracking research on a large number of children’s safety of live vaccination and other blood test indexes that can determine immunity is necessary.
This study has several limitations. First, the number of subjects was relatively small, and second, all of subjects had CD and there was no UC patient. Third, there was no control group who had taken the therapy only using the nonbiologic agent such as 5-aminosalicylic acid during pregnancy. To overcome these limitations, further prospective study subjecting a bigger group of reproductive-aged IBD woman who might keep their follow-ups during the pregnancy and also subjecting their children should be necessary.
In summary, this was the first study on Korean IBD women who gave birth after anti-TNF-α therapy during pregnancy even including their children’s immunity. IBD women who had anti-TNF-α during pregnancy did not show significant perinatal complications when they maintained remission state even in immunosuppressive combination therapy. From the clinical history about vaccination and anti-HBs titers, immunity of children born from IBD women who had been treated with anti-TNF-α medication during pregnancy seemed to be intact.
Go to : 
Notes
FINANCIAL SUPPORT
This work was supported by the research fund from the Korean Association for the Study of Intestinal Diseases (grant KASID-2014-04).
AUTHOR CONTRIBUTION
Conceptualization and design the study: Jung SA. Data collection, analysis and interpretation: Lee KE, Park SH, Moon CM, Shim SY, Cho SJ, Cho KB. Supervision: Jung SA, Kim SE, Kim ES, Yang SK. Writing - original draft: Lee KE. Writing - review and editing: Jung SA, Yang SK. Approval of final manuscript: all authors.
Go to : 
REFERENCES
1. Loftus EV Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002; 31:1–20.

2. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008; 14:542–549.

3. Kim HJ, Hann HJ, Hong SN, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis. 2015; 21:623–630.

4. Bush MC, Patel S, Lapinski RH, Stone JL. Perinatal outcomes in inflammatory bowel disease. J Matern Fetal Neonatal Med. 2004; 15:237–241.

5. Katz JA, Antoni C, Keenan GF, Smith DE, Jacobs SJ, Lichtenstein GR. Outcome of pregnancy in women receiving infliximab for the treatment of Crohn’s disease and rheumatoid arthritis. Am J Gastroenterol. 2004; 99:2385–2392.

6. Nørgård B, Hundborg HH, Jacobsen BA, Nielsen GL, Fonager K. Disease activity in pregnant women with Crohn’s disease and birth outcomes: a regional Danish cohort study. Am J Gastroenterol. 2007; 102:1947–1954.

7. Reddy D, Murphy SJ, Kane SV, Present DH, Kornbluth AA. Relapses of inflammatory bowel disease during pregnancy: inhospital management and birth outcomes. Am J Gastroenterol. 2008; 103:1203–1209.

8. Mahadevan U, Cucchiara S, Hyams JS, et al. The London Position Statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol. 2011; 106:214–223.

9. Schulze H, Esters P, Dignass A. Review article: the management of Crohn’s disease and ulcerative colitis during pregnancy and lactation. Aliment Pharmacol Ther. 2014; 40:991–1008.

10. Ng SW, Mahadevan U. My treatment approach to management of the pregnant patient with inflammatory bowel disease. Mayo Clin Proc. 2014; 89:355–360.

11. McConnell RA, Mahadevan U. Pregnancy and the patient with inflammatory bowel disease: fertility, treatment, delivery, and complications. Gastroenterol Clin North Am. 2016; 45:285–301.
12. Morales M, Berney T, Jenny A, Morel P, Extermann P. Crohn’s disease as a risk factor for the outcome of pregnancy. Hepatogastroenterology. 2000; 47:1595–1598.
13. Dominitz JA, Young JC, Boyko EJ. Outcomes of infants born to mothers with inflammatory bowel disease: a population-based cohort study. Am J Gastroenterol. 2002; 97:641–648.

14. Mottet C, Juillerat P, Pittet V, et al. Pregnancy and breastfeeding in patients with Crohn’s disease. Digestion. 2007; 76:149–160.

15. Ferguson CB, Mahsud-Dornan S, Patterson RN. Inflammatory bowel disease in pregnancy. BMJ. 2008; 337:a427.

16. Vermeire S, Carbonnel F, Coulie PG, et al. Management of inflammatory bowel disease in pregnancy. J Crohns Colitis. 2012; 6:811–823.

17. van der Woude CJ, Kolacek S, Dotan I, et al. European evidenced-based consensus on reproduction in inflammatory bowel disease. J Crohns Colitis. 2010; 4:493–510.

18. Dretzke J, Edlin R, Round J, et al. A systematic review and economic evaluation of the use of tumour necrosis factor-alpha (TNF-alpha) inhibitors, adalimumab and infliximab, for Crohn’s disease. Health Technol Assess. 2011; 15:1–244.
19. D’Haens GR, Panaccione R, Higgins PD, et al. The London Position Statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J astroenterol. 2011; 106:199–212.

20. Food and Drug Administration. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Final rule. Fed Regist. 2014; 79:72063–72103.
21. Department of Health Therapeutic Goods Administration. Australian categorisation system for prescribing medicines in pregnancy. Australian Government Web site. https://www.tga.gov.au/australian-categorisation-system-prescribing-medicines-pregnancy. Updated May 4, 2011. Accessed November 26, 2018.
22. Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996; 36:248–255.

23. Kane SV, Acquah LA. Placental transport of immunoglobulins: a clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. Am J Gastroenterol. 2009; 104:228–233.

25. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006; 4:621–630.

26. Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013; 11:286–292.

27. Vasiliauskas EA, Church JA, Silverman N, Barry M, Targan SR, Dubinsky MC. Case report: evidence for transplacental transfer of maternally administered infliximab to the newborn. Clin Gastroenterol Hepatol. 2006; 4:1255–1258.

28. Zelinkova Z, de Haar C, de Ridder L, et al. High intra-uterine exposure to infliximab following maternal anti-TNF treatment during pregnancy. Aliment Pharmacol Ther. 2011; 33:1053–1058.

29. Carter JD, Ladhani A, Ricca LR, Valeriano J, Vasey FB. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009; 36:635–641.

30. El Mourabet M, El-Hachem S, Harrison JR, Binion DG. Anti-TNF antibody therapy for inflammatory bowel disease during pregnancy: a clinical review. Curr Drug Targets. 2010; 11:234–241.

31. Gisbert JP. Safety of immunomodulators and biologics for the treatment of inflammatory bowel disease during pregnancy and breast-feeding. Inflamm Bowel Dis. 2010; 16:881–895.

32. Mahadevan U, Martin CF, Sandler RS, et al. PIANO: a 1000 patient prospective registry of pregnancy of pregnancy outcomes in women with IBD exposed to immunomodulators and biologic therapy. Gastroenterology. 2012; 142:S–149.
33. Julsgaard M, Christensen LA, Gibson PR, et al. Adalimumab and infliximab level in neonates. Gastroenterology. 2015; 148:S–108.
34. KOSIS. Review & assessment results for delivery: classification by birth weight. Statistics Korea Web site. http://kostat.go.kr/portal/korea/index.action. Accessed November 26, 2018.
35. An YW, Chung EH, Rheem I. A study of the current (2003-2005) prevalence of anti-HBs and immunologic memory of hepatitis B vaccine in children from the central area of Korea. Korean J Pediatr. 2006; 49:630–634.

36. Sheibani S, Cohen R, Kane S, Dubinsky M, Church JA, Mahadevan U. The effect of maternal peripartum anti-TNFalpha use on infant immune response. Dig Dis Sci. 2016; 61:1622–1627.

37. Beaulieu DB, Ananthakrishnan AN, Martin C, Cohen RD, Kane SV, Mahadevan U. Use of biologic therapy by pregnant women with inflammatory bowel disease does not affect infant response to vaccines. Clin Gastroenterol Hepatol. 2018; 16:99–105.

38. Gisbert JP, Chaparro M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol. 2013; 108:1426–1438.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download