Abstract
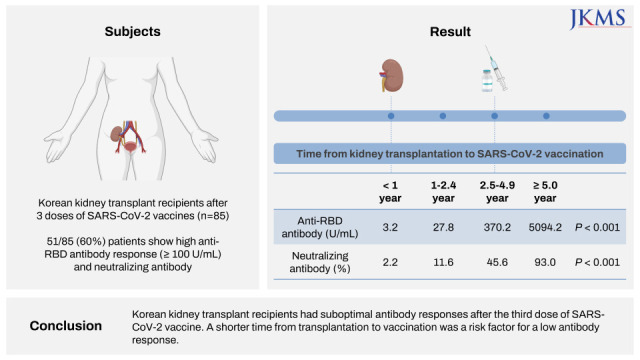
Eighty-five Korean kidney transplant recipients who received three doses of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine were tested with anti-receptor binding domain (RBD) antibody and neutralizing antibody. High anti-RBD antibody (≥ 100 U/mL) and neutralizing antibody responses (≥ 30%) were detected in 51/85 (60.0%) patients. When we divided the patients with the time from transplantation to vaccination (< 1, 1–2.4, 2.5–4.9, and ≥ 5-year), anti-RBD antibody titers were 3.2 U/mL, 27.8 U/mL, 370.2 U/mL, and 5,094.2 U/mL (P < 0.001) and anti-neutralizing antibody levels were 2.2%, 11.6%, 45.6%, and 93.0% (P < 0.001), respectively. Multivariate analysis revealed increased antibody responses when the time from transplantation to vaccination was five years or longer (odds ratio, 12.0; confidence interval, 2.7–52.8). Korean kidney transplant recipients had suboptimal antibody responses after the third dose of SARS-CoV-2 vaccine. A shorter time from transplantation to vaccination was a risk factor for a low antibody response.
Graphical Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody responses after the vaccination of kidney transplant recipients are significantly lower than in the normal population. Healthy individuals reach a seroconversion rate of over 99% after the second dose of SARS-CoV-2 vaccination, whereas kidney transplant recipients’ seroconversion rate was reported to be 17–58% after a second dose, and 55–69% after a third dose.1234 In addition, transplant recipients have a poor prognosis following SARS-CoV-2 infection. Solid organ transplant recipients have a 30% or greater risk of ventilation or death when infected with SARS-CoV-2 compared to a transplant naïve population, with a hospital admission rate over 50–80% and intensive care unit admission rate of 30%.5678
Although immunocompromised patients comprise a small proportion of the general population, they account for more than 40% of hospitalized breakthrough cases,9 with prolonged shedding of the virus resulting in persistent unintended exposure to others, as well as a potential source of new viral mutations.1011 Therefore, antibody responses in transplant recipients require great attention in terms of the efficient distribution of medical resources and increased risk of spreading infection.
As SARS-CoV-2 vaccination continues, studies evaluating immunogenicity in various immunocompromised groups are accumulating. However, not many studies have analyzed a sufficient number of patients.12 Especially studies of Asian transplantation recipients are limited.131415
In this study, we performed antibody tests after the third dose of SARS-CoV-2 vaccination in Korean kidney transplant recipients, and we analyzed factors correlated with low antibody production.
Patients who underwent kidney transplantation at Ewha Womans University Hospital (Mokdong Hospital and Seoul Hospital) and received three doses of SARS-CoV-2 vaccine were enrolled. Patients with a history of coronavirus disease 2019 or with positive anti-nucleocapsid antibody were excluded. Patients received available vaccine brands, according to the Korean national vaccination program. These vaccines included an mRNA vaccine (BNT162b2 [Pfizer-BioNTech] and mRNA-1273 [Moderna]), and viral vector vaccine (ChAdOx1 nCoV-19 [Oxford-AstraZeneca] and Ad26.COV2.S [Johnson and Johnson, Janssen]). Patient demographics, transplantation history, maintenance immunosuppression, time from transplant (TPL) to first vaccination (TPL to vaccine time), and time from last vaccination and antibody assays were collected. Kidney transplant recipients received immunosuppressive therapy according to our center’s protocol. Most recipients received triple immunosuppression therapy involving a calcineurin inhibitor, antimetabolites (mycophenolate mofetil and mycophenolate sodium), and steroids.
Patient samples were drawn between December 2021 and June 2022, at least two weeks after vaccination. These samples were tested with anti-nucleocapsid antibody (Elecsys anti-SARS-CoV-2; Roche Diagnostics, Mannheim, Germany), anti-receptor binding domain (RBD) antibody (Elecsys Anti-SARS-CoV-2 S; Roche Diagnostics), and neutralizing antibody (R-FIND SARS-CoV-2 Neutralizing Antibody ELISA; SG Medical, Seoul, Korea). High anti-RBD antibody responses were defined as anti-RBD antibody ≥ 100 U/mL. This cut-off was set based on the protected anti-RBD antibody titers in a challenge study, which was supported in a large clinical cohort as an upper value of the estimated level needed to provide 50% protective neutralization.1617 Positive neutralizing antibody responses were defined as ≥ 30% neutralization as per the manufacturer’s instructions.
The association between antibody responses with demographic and clinical characteristics was evaluated. For categorical variables, the χ2 test or Fisher’s test were performed. Continuous variables were assessed by the analysis of variance and are presented as the mean ± standard deviation. To assess factors associated with high anti-RBD antibody responses and the likelihood of occurrence according to clinical features, the odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using logistic regression analysis with forward selection. According to the TPL to vaccine time, the patients were divided into four groups: TPL to vaccine time < 1-year group, 1–2.4-year group, 2.5–4.9-year group, and ≥ 5-year group. Comparisons between these groups were performed. Analyses were performed with Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA), SPSS version 19.0 (IBM Corp., Armonk, NY, USA), SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and MedCalc version 20.027 (MedCalc Software, Ostend, Belgium).
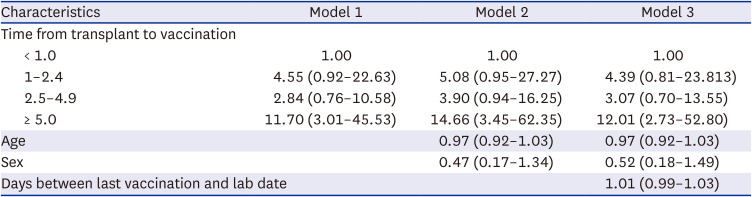
Eighty-five patients were enrolled. The baseline characteristics of the patients are shown in Table 1. Both high anti-RBD antibody and neutralizing antibody responses were detected in 51 patients (60.0%, 51/85). According to univariate analysis, time from transplantation to vaccination and time from last vaccination and antibody assay were associated with high anti-RBD antibody responses. Multivariate logistic analysis revealed an increased likelihood of high anti-RBD antibody responses when the duration of transplantation to vaccination was five years or more (OR, 12.01; CI, 2.73–52.80) (Table 2).
The high anti-RBD antibody response rate and mean anti-RBD antibody titer were significantly different between the TPL to vaccine time < 1-year, 1–2.4-year, 2.5–4.9-year, and ≥ 5-year groups. High anti-RBD antibody response rate were 9.8%, 13.7%, 23.5%, and 52.9% (P = 0.002) and mean anti-RBD antibody titer were 3.2 U/mL, 27.8 U/mL, 370.2 U/mL, and 5,094.2 U/mL (P < 0.001) (Fig. 1). The neutralizing antibody response rate and mean neutralizing antibody % were significantly different between the TPL to vaccine time < 1-year, 1–2.4-year, 2.5–4.9-year, and ≥ 5-year groups. Neutralizing antibody response rate were 0%, 0%, 78.3%, and 100% (P < 0.001) and mean neutralizing antibody % were 2.2%, 11.6%, 45.6%, and 93.0% (P < 0.001) (Fig. 1). The OR of the chances of developing high anti-RBD antibodies increased as the time between transplantation and the first dose of vaccination increased (Fig. 2).
In this study high anti-RBD antibody responses were observed in 60% of Korean kidney transplant recipients, who received three doses of SARS-CoV-2 vaccines. This was similar to a previous report where three-dose vaccinated solid organ transplant recipients had an antibody response rate of 67% (interquartile range. 55–69%).2 When high anti-RBD antibody responses were compared in Korean healthy subjects, the antibody response in kidney transplant recipients was significantly lower. According to a study conducted on Korean healthcare workers, 100% of the subjects showed a high anti-RBD response of 100 U/mL or more.18 The antibody response rate of kidney transplant recipients was lower than that of end-stage renal disease patients undergoing maintenance hemodialysis, who reached 83–96% positive results of neutralizing antibody.19
A shorter time from transplantation to vaccination was an independent risk factor for a low or negative anti-RBD antibody response. The high anti-RBD antibody response rate was significantly higher in the TPL to vaccine time ≥ 5-year group with an OR of 12.0. The OR of developing anti-RBD antibodies increased as the time between transplantation and vaccination increased. This is probably because patients who were at the furthest point from the introduction treatment are generally maintained with lower immunosuppression.13 The first 3–6 months post-transplantation is widely known as the period of maximum immunosuppression with evidence pointing to a lower vaccine response during this period of intense immunosuppression. Previous studies also reported that a shorter time from transplantation to vaccination was associated with negative antibody responses,2021 but the threshold time point from transplantation to vaccination differed between studies, ranging from six months to four years.1322
In addition to a shorter time from transplantation to vaccination, previous studies report that older age, steroid treatment, antimetabolite treatment, higher waist circumference, and comorbidities such as hypertension were associated with reduced antibody responses in transplant recipients.212223 Our medical center uses steroids continuously rather than withdrawing them. Therefore, most of the study subjects (81.2%) were treated with steroids, and the effect of this variable was diluted due to an insufficient number of subjects. Recently, Kang et al.24 reported that two doses of mixed vaccination (ChAdOx1 nCoV-19 and BNT162b2) had a higher antibody response than the matched adenovirus vector vaccine and had a similar antibody response with the matched mRNA vaccine in Korean solid organ transplant recipients. However, there were no differences according to the number of viral vector vaccines used in this study.
The limitation of this study is that the sample size was relatively small when variable groups were stratified. In addition, there was no control group. This study used a cut-off value of 100 U/mL to determine high antibody responses, which is arbitrary and does not necessarily predict resistance to infection.
In conclusion, Korean kidney transplantation recipients had suboptimal antibody responses after three doses of SARS-CoV-2 vaccination. Time from transplantation to vaccination was an independent risk factor. We believe that the accumulation of these studies will contribute to the optimization of the SARS-CoV-2 vaccination protocol for kidney transplant recipients. Further studies on immunocompromised patients and ethnicity effects on immunogenicity are needed.
The present study protocol was reviewed and approved by the Institutional Review Board of Ewha Womans University Mokdong Hospital (approval number: EUMC 2021-10-043) and Institutional Review Board Ewha Womans University Seoul Hospital (approval number: SEUMC 2021-12-006). Informed consent was submitted by all subjects when they were enrolled.
Notes
Author Contributions:
Conceptualization: Song SH, Chung KY, Kim SK.
Data curation: Song SH, Chung KY, Chung HS, Kim K, Minn D, Kim SK.
Formal analysis: Song SH, Jee Y, Kim SK.
Investigation: Song SH, Chung KY, Chung HS, Kim K, Minn D, Kim SK.
Methodology: Song SH, Kim SK.
Software: Jee Y, Kim SK.
Validation: Song SH, Kim SK.
Visualization: Jee Y, Kim SK.
Writing - original draft: Song SH, Jee Y, Kim SK.
Writing - review & editing: Song SH, Chung KY, Chung HS, Kim K, Minn D, Kim SK.
References
1. Wei J, Stoesser N, Matthews PC, Ayoubkhani D, Studley R, Bell I, et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021; 6(9):1140–1149. PMID: 34290390.
2. Parker EP, Desai S, Marti M, Nohynek H, Kaslow DC, Kochhar S, et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health. 2022; 10(3):e326–e328. PMID: 35180408.
3. Lee AR, Wong SY, Chai LY, Lee SC, Lee MX, Muthiah MD, et al. Efficacy of COVID-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022; 376:e068632. PMID: 35236664.
4. Tsapepas D, Paget K, Mohan S, Cohen DJ, Husain SA. Clinically significant COVID-19 following SARS-CoV-2 vaccination in kidney transplant recipients. Am J Kidney Dis. 2021; 78(2):314–317. PMID: 34019949.
5. Raja MA, Mendoza MA, Villavicencio A, Anjan S, Reynolds JM, Kittipibul V, et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando). 2021; 35(1):100588. PMID: 33246166.
6. Nair V, Jandovitz N, Hirsch JS, Abate M, Satapathy SK, Roth N, et al. An early experience on the effect of solid organ transplant status on hospitalized COVID-19 patients. Am J Transplant. 2021; 21(7):2522–2531. PMID: 33443778.
7. Sutharattanapong N, Thotsiri S, Kantachuvesiri S, Wiwattanathum P. Benefits of inactivated vaccine and viral vector vaccine immunization on COVID-19 infection in kidney transplant recipients. Vaccines (Basel). 2022; 10(4):572. PMID: 35455322.
8. Kang JM, Kim YJ, Huh K, Kim JM, Park WB, Ahn HJ, et al. COVID-19 among solid organ transplant recipients in Korea: surveillance data of the Korean Transplantation Society, January 2020 to March 2022. Korean J Transplant. 2022; 36(2):159–163. PMID: 35919200.
9. Parker EPK, Desai S, Marti M, Nohynek H, Kaslow DC, Kochhar S, et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health. 2022; 10(3):e326–e328. PMID: 35180408.
10. Ribas A, Sengupta R, Locke T, Zaidi SK, Campbell KM, Carethers JM, et al. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov. 2021; 11(2):233–236. PMID: 33355178.
11. Aydillo T, Gonzalez-Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020; 383(26):2586–2588. PMID: 33259154.
12. Efros O, Anteby R, Halfon M, Meisel E, Klang E, Soffer S. Efficacy and safety of third dose of the COVID-19 vaccine among solid organ transplant recipients: a systemic review and meta-analysis. Vaccines (Basel). 2022; 10(1):95. PMID: 35062756.
13. Yi SG, Moore LW, Eagar T, Graviss EA, Nguyen DT, Ibrahim H, et al. Risk factors associated with an impaired antibody response in kidney transplant recipients following 2 doses of the SARS-CoV-2 mRNA vaccine. Transplant Direct. 2021; 8(1):e1257. PMID: 34912946.
14. Boyarsky BJ, Werbel WA, Avery RK, Tobian AA, Massie AB, Segev DL, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021; 325(21):2204–2206. PMID: 33950155.
15. Boyarsky BJ, Werbel WA, Avery RK, Tobian AA, Massie AB, Segev DL, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021; 325(17):1784–1786. PMID: 33720292.
16. Hall VG, Ferreira VH, Ku T, Ierullo M, Majchrzak-Kita B, Chaparro C, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021; 385(13):1244–1246. PMID: 34379917.
17. Bertrand D, Hamzaoui M, Lemée V, Lamulle J, Laurent C, Etienne I, et al. Antibody and T-cell response to a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Kidney Int. 2021; 100(6):1337–1340. PMID: 34619232.
18. Kim N, Minn D, Park S, Roh EY, Yoon JH, Park H, et al. Positivity of SARS-CoV-2 antibodies among Korean healthy healthcare workers 1 and 2 weeks after second dose of Pfizer-BioNTech vaccination. J Korean Med Sci. 2021; 36(21):e158. PMID: 34060264.
19. Park JS, Minn D, Hong S, Jeong S, Kim S, Lee CH, et al. Immunogenicity of COVID-19 vaccination in patients with end-stage renal disease undergoing maintenance hemodialysis: the efficacy of a mix-and-match strategy. J Korean Med Sci. 2022; 37(23):e180. PMID: 35698835.
20. Carr EJ, Kronbichler A, Graham-Brown M, Abra G, Argyropoulos C, Harper L, et al. Review of early immune response to SARS-CoV-2 vaccination among patients with CKD. Kidney Int Rep. 2021; 6(9):2292–2304. PMID: 34250319.
21. Balsby D, Nilsson AC, Möller S, Lindvig SO, Davidsen JR, Abazi R, et al. Determinants of antibody response to a third SARS-CoV-2 mRNA vaccine dose in solid organ transplant Recipients: results from the prospective cohort study COVAC-Tx. Vaccines (Basel). 2022; 10(4):565. PMID: 35455314.
22. Masset C, Kerleau C, Garandeau C, Ville S, Cantarovich D, Hourmant M, et al. A third injection of the BNT162b2 mRNA COVID-19 vaccine in kidney transplant recipients improves the humoral immune response. Kidney Int. 2021; 100(5):1132–1135. PMID: 34474075.
23. Watanabe M, Balena A, Tuccinardi D, Tozzi R, Risi R, Masi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab Res Rev. 2022; 38(1):e3465. PMID: 33955644.
24. Kang JM, Lee J, Huh KH, Joo DJ, Lee JG, Kim HR, et al. Matched versus mixed COVID-19 vaccinations in korean solid organ transplant recipients: an Observational Study. Transplantation. 2022; 106(9):e392–e403. PMID: 35749755.
Fig. 1
Antibody responses in kidney transplant recipients. (A) Anti-RBD antibody titers were significantly different according to the time from transplantation to vaccination. (B) Neutralizing antibody levels were significantly different according to the time from transplantation to vaccination.
RBD = receptor binding domain.
***P < 0.001
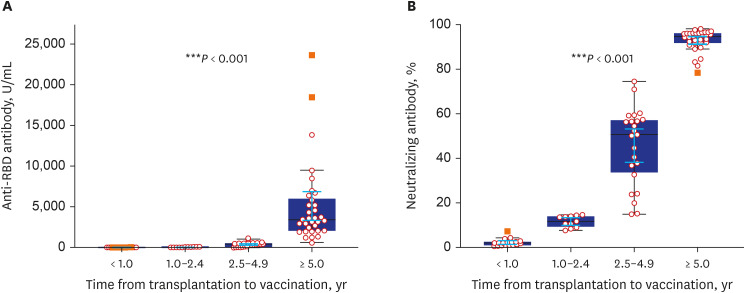
Fig. 2
Odds ratio (y-axis) of the chances of developing anti-RBD antibodies (≥ 100 U/mL) according to the time from transplantation to vaccination.
RBD = receptor binding domain.
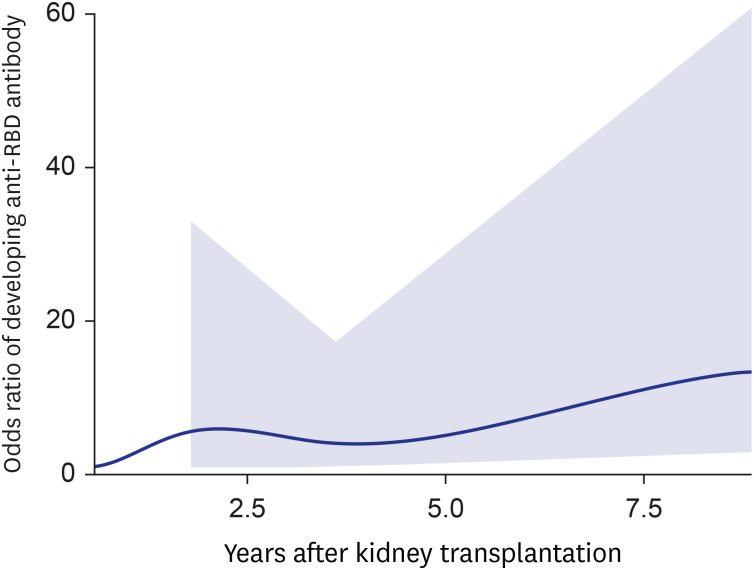
Table 1
Demographics and antibody responses of kidney transplant recipients who completed three doses of SARS-CoV-2 vaccine
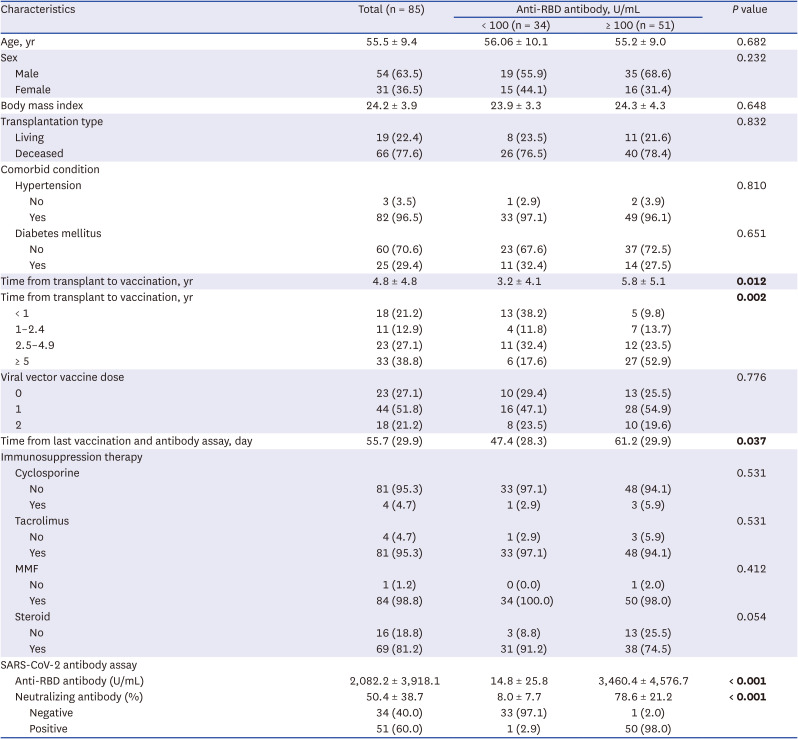
Table 2
The multivariate-adjusted odds ratios for high anti-receptor binding domain antibody responses (≥ 100 U/mL)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download