INTRODUCTION
MATERIALS AND METHODS
Chemicals and reagent
Preparation of ethanol extracts of CF or SF, and their mixture
Animals and treatment
Blood analysis
Histological analysis and immunohistochemistry (IHC)
Serum analysis for 5α-reductase type 2, DHT, testosterone, and PSA
Statistical analysis
RESULTS
Mixture of CF and SF (MIX) attenuates the prostate weights and volumes of TP-induced BPH rats
 | Fig. 1Effects of ethanol extracts of CF and SF, and their mixture (MIX) on prostate weight and volume in TP-induced BPH rats. The rats with BPH induced by TP (3 mg/kg) were treated with SP(45 mg/kg), finasteride (Fina, 5 mg/kg) as a PC, CF (50 mg/kg), SF (50 mg/kg), and MIX (50 mg/kg). Changes in prostate weight (A), prostate index (B), and width of VP (C). Data are expressed as the mean ± SD (n = 6). (D) Representative photographs of prostate tissue in each group.Con group, corn oil injection and DW fed group; BPH group, TP injection and DW fed group; SP group, TP injection and saw palmetto fed group; PC group, TP and finasteride injection group; CF group, TP injection and CF fed group; SF group, TP injection and SF fed group; MIX group, TP injection and SF/CF mixture fed group; BPH, benign prostatic hyperplasia; SP, saw palmetto; PC, positive control; CF, Corni Fructus; SF, Schisandrae Fructus; AP, anterior prostate lobe; DP, dorsal prostate lobe; VP, ventral prostate lobe; TP, testosterone propionate; DW, distilled water.
***P < 0.001 compared to the control group. ##P < 0.01 and ###P < 0.001 compared to the BPH group.
|
MIX improves the histological changes in TP-induced BPH rats
 | Fig. 2Effects of CF, SF, and MIX on histological changes in TP-induced BPH rats. Representative images of hematoxylin and eosin-stained prostate (A) and testis (B) tissue are shown. Scale bar = 100 μm. (C) The lumen area of the prostate tissue in each group was measured. (D) The thickness of epithelium tissue from prostate in each group was evaluated. Each value indicates the mean ± SD (n = 6).Con group, corn oil injection and DW fed group; BPH group, TP injection and DW fed group; SP group, TP injection and saw palmetto fed group; PC group, TP and finasteride injection group; CF group, TP injection and CF fed group; SF group, TP injection and SF fed group; MIX group, TP injection and SF/CF mixture fed group; BPH, benign prostatic hyperplasia; SP, saw palmetto; PC, positive control; CF, Corni Fructus; SF, Schisandrae Fructus; TP, testosterone propionate; DW, distilled water.
***P < 0.001 compared to the control group. ##P < 0.01 and ###P < 0.001 compared to the BPH group.
|
MIX regulates the levels of 5α-reductase type 2, testosterone, and DHT in TP-induced BPH rats
 | Fig. 3Effects of CF, SF, and MIX on the expression and concentration of 5α-reductase type 2 in TP-induced BPH rats. (A) Expression of 5α-reductase type 2 in prostate tissues of each group was determined by immunohistochemistry. Scale bar = 100 μm. (B) 5α-reductase type 2 expression was quantified in the regions of each group. The levels of SRD5A2 (C), testosterone (D) and DHT (E) in serum of each group were detected through ELISA analysis. Each value indicates the mean ± SD (n = 6).Con group, corn oil injection and DW fed group; BPH group, TP injection and DW fed group; SP group, TP injection and saw palmetto fed group; PC group, TP and finasteride injection group; CF group, TP injection and CF fed group; SF group, TP injection and SF fed group; MIX group, TP injection and SF/CF mixture fed group; BPH, benign prostatic hyperplasia; SP, saw palmetto; PC, positive control; CF, Corni Fructus; SF, Schisandrae Fructus; SRD5A2, 5α-reductase type 2; DHT, dihydrotestosterone; TP, testosterone propionate; DW, distilled water.
***P < 0.001 compared to the control group. #P < 0.05, ##P < 0.01 and ###P < 0.001 compared to the BPH group.
|
MIX suppresses the levels of PSA in TP-induced BPH rats
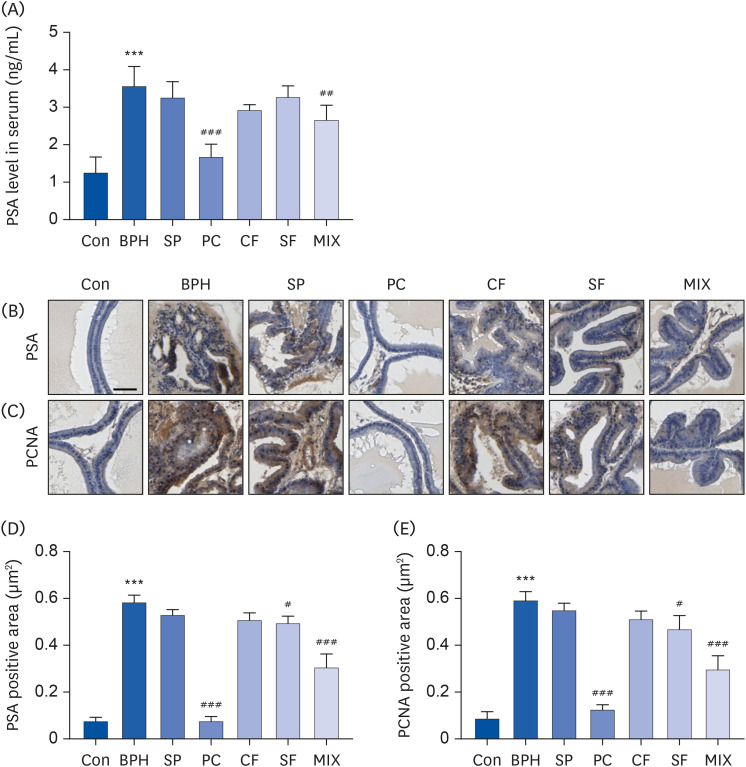 | Fig. 4Effects of CF, SF and MIX on the expression of PSA and PCNA in TP-induced BPH rats. (A) The serum concentrations of PSA in each group were examined by ELISA. (B and C) The expression of PSA and PCNA in prostate tissues of each group examined by immunohistochemistry. Scale bar = 50 μm. (D and E) The expression of PSA and PCNA in prostate tissues was quantified. Data are expressed as the mean ± SD (n = 6).Con group, corn oil injection and DW fed group; BPH group, TP injection and DW fed group; SP group, TP injection and saw palmetto fed group; PC group, TP and finasteride injection group; CF group, TP injection and CF fed group; SF group, TP injection and SF fed group; MIX group, TP injection and SF/CF mixture fed group; PSA, prostate-specific antigen; BPH, benign prostatic hyperplasia; SP, saw palmetto; PC, positive control; CF, Corni Fructus; SF, Schisandrae Fructus; PCNA, proliferating cell nuclear antigen; TP, testosterone propionate; DW, distilled water.
***P < 0.001 compared to the control group. ##P < 0.01 and ###P < 0.001 compared to the BPH group.
|
MIX regulates the AR signaling pathway-related proteins in TP-induced BPH rats
 | Fig. 5Effects of CF, SF, and MIX on AR and its co-activators in prostate tissues of TP-induced BPH rats. To evaluate the expression of AR, ARA70, and SRC1, paraffin blocks of prostate tissue of each group were sectioned into 4 μm in thickness and stained with specific antibodies (n = 6). Representative images of prostate tissues immunostained with an anti-AR (A), ARA70 (B), SRC1 (C), and α-SMA (D) are presented. Scale bar = 50 μm. Quantifications of immunohistochemistry staining of AR (E), ARA70 (F), SRC1 (G), and α-SMA (H). Data are expressed as the mean ± SD (n = 6).Con group, corn oil injection and DW fed group; BPH group, TP injection and DW fed group; SP group, TP injection and saw palmetto fed group; PC group, TP and finasteride injection group; CF group, TP injection and CF fed group; SF group, TP injection and SF fed group; MIX group, TP injection and SF/CF mixture fed group; BPH, benign prostatic hyperplasia; SP, saw palmetto; PC, positive control; CF, Corni Fructus; SF, Schisandrae Fructus; AR, androgen receptor; ARA70, AR coactivator 70; SRC1, steroid receptor coactivator 1; α-SMA, α-smooth muscle actin; TP, testosterone propionate; DW, distilled water.
***P < 0.001 compared to the control group. ##P < 0.01 and ###P < 0.001 compared to the BPH group.
|
MIX modulates the expression of growth factors and apoptosis-related proteins in TP-induced BPH rats
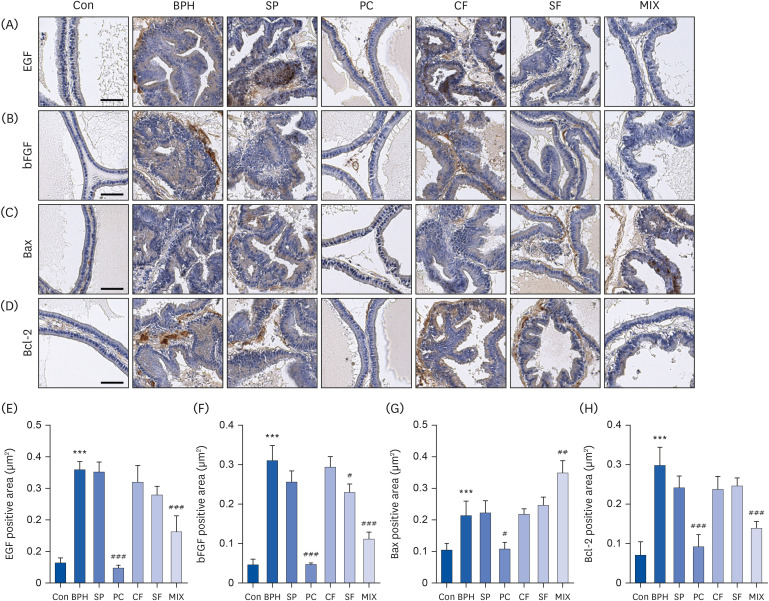 | Fig. 6Effects of CF, SF, and MIX on expression of growth factors, Bax, and Bcl-2 in prostate tissues of TP-induced BPH rats. After treatment with TP and/or chemicals for 7 wk, the prostate tissues were obtained and their paraffin blocks of prostate tissues were sectioned into 4 μm in thickness and stained with specific antibodies. The prostatic expression levels of epidermal growth factor, basic fibrosis growth factor, Bax, and Bcl-2 were detected by immunohistochemistry (n = 6). Scale bar = 50 μm. Quantifications of IHC staining of EGF (E), bFGF (F), Bax (G), and Bcl-2 (H). Data are expressed as the mean ± SD (n = 6).Con group, corn oil injection and DW fed group; BPH group, TP injection and DW fed group; SP group, TP injection and saw palmetto fed group; PC group, TP and finasteride injection group; CF group, TP injection and CF fed group; SF group, TP injection and SF fed group; MIX group, TP injection and SF/CF mixture fed group; BPH, benign prostatic hyperplasia; SP, saw palmetto; PC, positive control; CF, Corni Fructus; SF, Schisandrae Fructus; TP, testosterone propionate; EGF, epidermal growth factor; bFGF, basic fibroblast growth factor; DW, distilled water.
***P < 0.001 compared to the control group. ##P < 0.01 and ###P < 0.001 compared to the BPH group.
|
MIX does not induce toxicity in TP-induced BPH rats
Table 1
Effects of CF, SF and MIX treatment on the organ weights of BPH rats
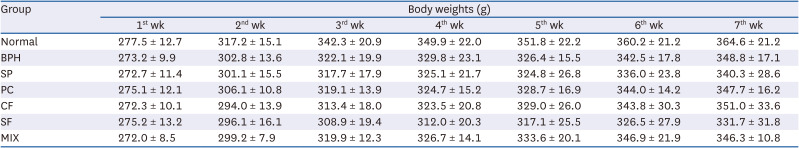
Table 2
Effects of CF, SF and MIX treatment on the organ weights of BPH rats
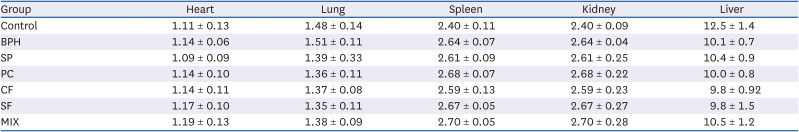
Table 3
Effects of CF, SF and MIX treatment on the serum biochemical analysis of BPH rats
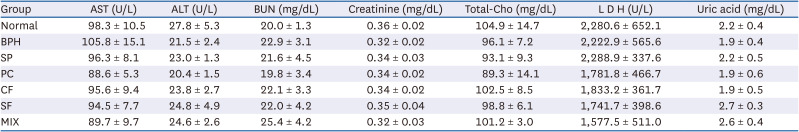
Table 4
Effects of CF, SF and MIX treatment on the hematological analysis of BPH rats
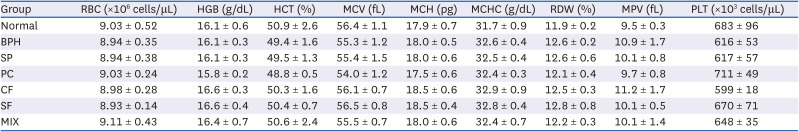




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download