INTRODUCTION
MATERIALS AND METHODS
Materials
Cell culture and sample treatment
Measurement of cell viability
Enzyme-linked immunosorbent assay (ELISA)
Evaluation of mRNA levels
Measurement of HAT and HDAC activity using ELISA
Immunoblot analysis
Immunoprecipitation assays
Statistical analysis
RESULTS
Cytotoxic effects of NaB in HG-treated THP-1 cells
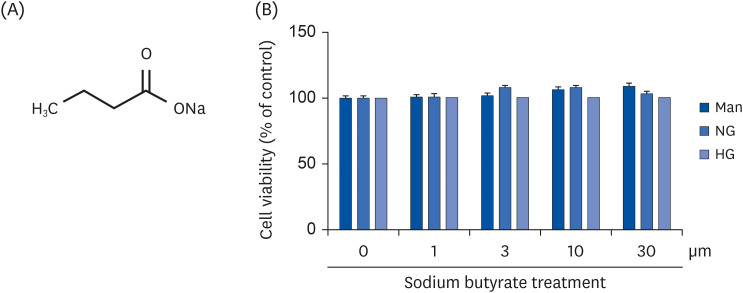 | Fig. 1Cytotoxicity of NaB to cultured HG-treated THP-1 cells. (A) Chemical structure of NaB. (B) The effect of NaB on the cell viability after 48 h was evaluated by the CCK-8 assay. Human monocytic THP-1 cells (1 × 105 cells/mL) were cultured in the presence of an osmolar control (14.5 mmol/L mannitol), under NG (5.5 mmol/L glucose) or HG (20 mmol/L glucose) conditions, or in the absence or presence of NaB (0, 1, 3, 10, 30 μM) for 48 h. The cell culture media was collected for the measurement of cytokine release. The data represent the mean ± SD of 3 independent experiments.CCK-8, Cell Counting Kit-8; NaB, sodium butyrate; Man, mannitol; NG, normoglycemia; HG, high glucose.
|
Effects of NaB on inflammatory cytokines production of HG-treated THP-1 cells
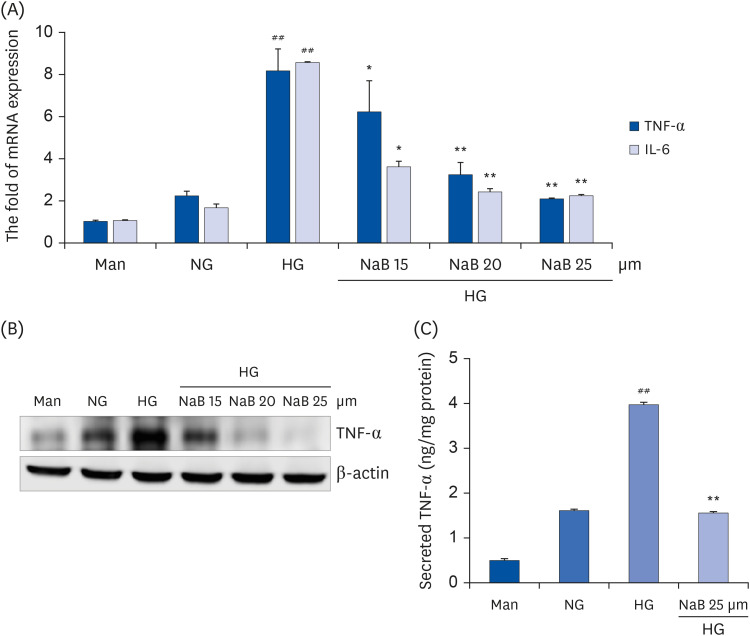 | Fig. 2NaB-mediated inhibition of cytokine release in HG-treated THP-1 cells. (A) Cells (1 × 105 cells/mL) were treated with NaB for 48 h, and the mRNA levels were evaluated by a quantitative real-time polymerase chain reaction. (B) Cell lysates were prepared, and TNF-α levels were evaluated by western blot analysis, as described in the Materials and Methods section. Equal protein loading was confirmed by stripping the immunoblot and reprobing it for β-actin protein. The immunoblots shown here are representative of 3 independent experiments. (C) TNF-α in the cell media was measured using an ELISA assay kit. The cytokine levels in the media were measured using an ELISA assay kit according to the manufacturer’s instructions. The values were calculated based on the assay’s standard curve. The data represent the mean ± SD of 3 independent experiments.NaB, sodium butyrate; Man, mannitol; NG, normoglycemia; HG, high glucose; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; ELISA, enzyme-linked immunosorbent assay.
##P < 0.01 compared with NG; *P < 0.05, **P < 0.01 compared with HG.
|
Modulatory effect of NaB on HAT and HDAC activity of HG-treated THP-1 cells
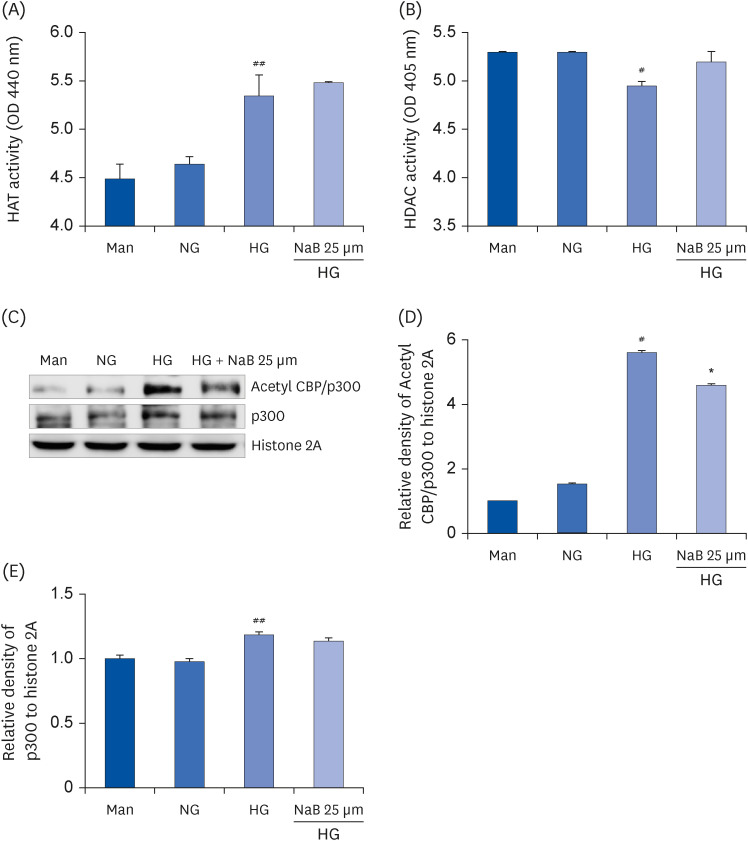 | Fig. 3Effect of NaB on the HAT and HDAC activity as well as p300 and Acetyl CBP/p300 levels in HG-treated THP-1 cells. The cells were harvested after 48 h of the NaB treatment, and nuclear lysates were prepared. The samples were analyzed to determine the HAT (A) and HDAC activity (B). After nuclear protein extraction, the p300 and Acetyl CBP/p300 levels (C) were evaluated by western blot analysis. Densities of (D) Acetyl CBP/p300, (E) p300 normalized to β-actin using ImageJ software. The data represent the mean ± SD of 3 independent experiments.NaB, sodium butyrate; Man, mannitol; NG, normoglycemia; HG, high glucose; HAT, histone acetyltransferase; HDAC, histone deacetylase; CBP/p300, CREB-binding protein/p300; OD, optical density; Acetyl, acetylated.
#P < 0.05, ##P < 0.01 compared with NG; *P < 0.05 compared with HG.
|
Effect of NaB on NF-κB p65 activation in HG-treated THP-1 cells
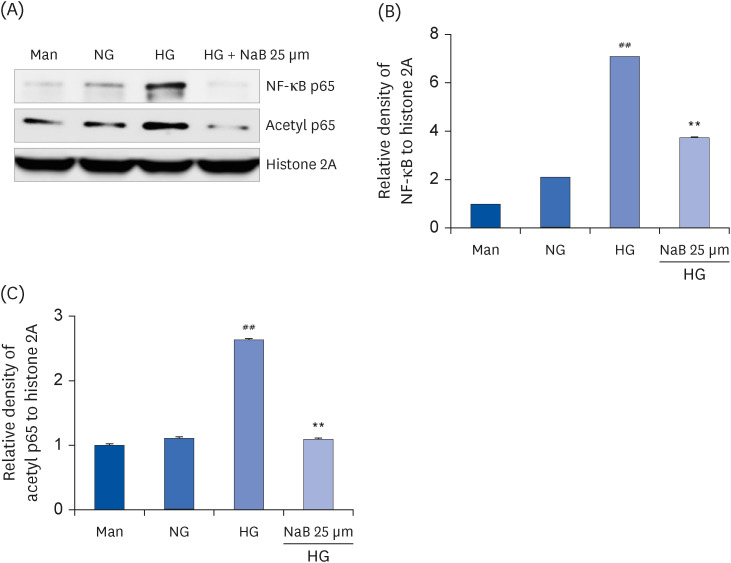 | Fig. 4NaB-induced suppression of NF-κB activation in HG-treated THP-1 cells. (A) The protein levels were evaluated by western blot for NF-κB p65 and Acetyl p65. An equal protein loading was confirmed by stripping the immunoblot and reprobing it for the histone 2A protein. Densities of (B) NF-κB p65, (C) Acetyl p65 normalized to β-actin using ImageJ software. The data represent the mean ± SD for 3 independent experiments.NaB, sodium butyrate; Man, mannitol; NG, normoglycemia; HG, high glucose; NF-κB, nuclear factor-κB; Acetyl, acetylated.
##P < 0.01 compared to NG; **P < 0.01 compared with HG.
|
Effect of NaB on the interaction between inflammation-involved genes and p300
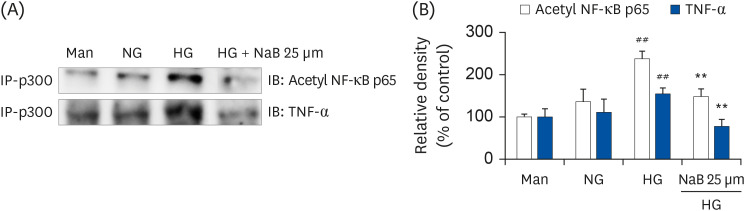 | Fig. 5NaB-induced interaction of p300 with Acetyl p65 and TNF-α in HG-treated THP-1 cells. (A) The cells were treated with NaB for 48 h, and nuclear lysates were prepared. p300 was immunoprecipitated, and the interaction with Acetyl p65 and TNF-α was assessed by western blotting. The immunoblots shown here are representative of 3 independent experiments. (B) Quantitative representation of Acetyl p65 and TNF-α in p300. The data represent mean ± SD for 3 independent experiments.NaB, sodium butyrate; Man, mannitol; NG, normoglycemia; HG, high glucose; NF-κB, nuclear factor-κB; TNF-α, tumor necrosis factor-α; Acetyl, acetylated.
##P < 0.01 compared with NG; **P < 0.01 compared with HG.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download