1. Cho J, Hong H, Park S, Kim S, Kang H. Insulin resistance and its association with metabolic syndrome in Korean children. BioMed Res Int. 2017; 2017:8728017. PMID:
29457038.
2. Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. 2013; 3:1–58. PMID:
23720280.
3. Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002; 420:333–336. PMID:
12447443.

4. Park MH, Kim DH, Lee EK, Kim ND, Im DS, Lee J, Yu BP, Chung HY. Age-related inflammation and insulin resistance: a review of their intricate interdependency. Arch Pharm Res. 2014; 37:1507–1514. PMID:
25239110.

5. Lillycrop KA, Burdge GC. Maternal diet as a modifier of offspring epigenetics. J Dev Orig Health Dis. 2015; 6:88–95. PMID:
25857738.
6. Weinhold B. Epigenetics: the science of change. Environ Health Perspect. 2006; 114:A160–A167. PMID:
16507447.

7. Yokomori N, Tawata M, Onaya T. DNA demethylation during the differentiation of 3T3-L1 cells affects the expression of the mouse GLUT4 gene. Diabetes. 1999; 48:685–690. PMID:
10102682.
8. Toperoff G, Aran D, Kark JD, Rosenberg M, Dubnikov T, Nissan B, Wainstein J, Friedlander Y, Levy-Lahad E, Glaser B, et al. Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Hum Mol Genet. 2012; 21:371–383. PMID:
21994764.
9. Kuroda A, Rauch TA, Todorov I, Ku HT, Al-Abdullah IH, Kandeel F, Mullen Y, Pfeifer GP, Ferreri K. Insulin gene expression is regulated by DNA methylation. PLoS One. 2009; 4:e6953. PMID:
19742322.
10. Zheng S, Rollet M, Pan YX. Maternal protein restriction during pregnancy induces CCAAT/enhancer-binding protein (C/EBPβ) expression through the regulation of histone modification at its promoter region in female offspring rat skeletal muscle. Epigenetics. 2011; 6:161–170. PMID:
20930553.

11. Bouchard L, Hivert MF, Guay SP, St-Pierre J, Perron P, Brisson D. Placental adiponectin gene DNA methylation levels are associated with mothers’ blood glucose concentration. Diabetes. 2012; 61:1272–1280. PMID:
22396200.

12. Jung BC, Kang S. Epigenetic regulation of inflammatory factors in adipose tissue. Biochim Biophys Acta Mol Cell Biol Lipids. 2021; 1866:159019. PMID:
34332076.

13. Lee HT, Oh S, Ro DH, Yoo H, Kwon YW. The key role of DNA methylation and histone acetylation in epigenetics of atherosclerosis. J Lipid Atheroscler. 2020; 9:419–434. PMID:
33024734.

14. Inoue A, Shen L, Dai Q, He C, Zhang Y. Generation and replication-dependent dilution of 5fC and 5caC during mouse preimplantation development. Cell Res. 2011; 21:1670–1676. PMID:
22124233.

15. Fritz EL, Papavasiliou FN. Cytidine deaminases: AIDing DNA demethylation? Genes Dev. 2010; 24:2107–2114. PMID:
20889711.

16. Liu J, Chen M, Wang X. Calcitonin gene-related peptide-enhanced nitric oxide release and inducible NOS activity and mRNA expression in LPS-stimulated mouse peritoneal macrophages. Shock. 2001; 16:64–69. PMID:
11442318.

17. Moon J, Koh G. Clinical evidence and mechanisms of high-protein diet-induced weight loss. J Obes Metab Syndr. 2020; 29:166–173. PMID:
32699189.

18. Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004; 23:373–385. PMID:
15466943.

19. van der Klaauw AA, Keogh JM, Henning E, Trowse VM, Dhillo WS, Ghatei MA, Farooqi IS. High protein intake stimulates postprandial GLP1 and PYY release. Obesity (Silver Spring). 2013; 21:1602–1607. PMID:
23666746.

20. Markova M, Pivovarova O, Hornemann S, Sucher S, Frahnow T, Wegner K, Machann J, Petzke KJ, Hierholzer J, Lichtinghagen R, et al. Isocaloric diets high in animal or plant protein reduce liver fat and inflammation in individuals with type 2 diabetes. Gastroenterology. 2017; 152:571–585.e8. PMID:
27765690.

21. Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989; 320:265–271. PMID:
2783477.
22. Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003; 77:1146–1155. PMID:
12716665.
23. Strauss MH, Dorian P, Verma S. Fish oil supplementation and arrhythmias. JAMA. 2005; 294:2165. PMID:
16264152.

24. Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996; 63:116–122. PMID:
8604658.

25. DiNicolantonio JJ, O’Keefe JH. Importance of maintaining a low omega-6/omega-3 ratio for reducing inflammation. Open Heart. 2018; 5:e000946. PMID:
30564378.
26. Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002; 56:365–379. PMID:
12442909.
27. Gatica LV, Oliveros LB, Pérez Díaz MF, Domínguez NS, Fornes MW, Gimenez MS. Implication of vitamin A deficiency on vascular injury related to inflammation and oxidative stress. Effects on the ultrastructure of rat aorta. Eur J Nutr. 2012; 51:97–106. PMID:
21512820.
28. Harshman SG, Shea MK. The role of vitamin K in chronic aging diseases: inflammation, cardiovascular disease, and osteoarthritis. Curr Nutr Rep. 2016; 5:90–98. PMID:
27648390.
29. Slusher AL, McAllister MJ, Huang CJ. A therapeutic role for vitamin D on obesity-associated inflammation and weight-loss intervention. Inflamm Res. 2015; 64:565–575. PMID:
26142253.
30. Alcalá M, Sánchez-Vera I, Sevillano J, Herrero L, Serra D, Ramos MP, Viana M. Vitamin E reduces adipose tissue fibrosis, inflammation, and oxidative stress and improves metabolic profile in obesity. Obesity (Silver Spring). 2015; 23:1598–1606. PMID:
26148343.
31. Lewis ED, Meydani SN, Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019; 71:487–494. PMID:
30501009.
32. Zhang Z, Liu S, Qi Y, Aluo Z, Zhang L, Yu L, Li Q, Luo Z, Sun Z, Zhou L, et al. Calcium supplementation relieves high-fat diet-induced liver steatosis by reducing energy metabolism and promoting lipolysis. J Nutr Biochem. 2021; 94:108645. PMID:
33838230.
33. Reyes-Garcia R, Mendoza N, Palacios S, Salas N, Quesada-Charneco M, Garcia-Martin A, Fonolla J, Lara-Villoslada F, Muñoz-Torres M. Effects of daily intake of calcium and vitamin D-enriched milk in healthy postmenopausal women: a randomized, controlled, double-blind nutritional study. J Womens Health (Larchmt). 2018; 27:561–568. PMID:
29676968.
34. Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008; 105:17046–17049. PMID:
18955703.
35. Wang Z, Yao T, Pini M, Zhou Z, Fantuzzi G, Song Z. Betaine improved adipose tissue function in mice fed a high-fat diet: a mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2010; 298:G634–G642. PMID:
20203061.
36. Guerrerio AL, Colvin RM, Schwartz AK, Molleston JP, Murray KF, Diehl A, Mohan P, Schwimmer JB, Lavine JE, Torbenson MS, et al. Choline intake in a large cohort of patients with nonalcoholic fatty liver disease. Am J Clin Nutr. 2012; 95:892–900. PMID:
22338037.
37. Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, Thurston A, Huntley JF, Rees WD, Maloney CA, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci U S A. 2007; 104:19351–19356. PMID:
18042717.
38. Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, Bhat DS, Naik SS, Coyaji KJ, Joglekar CV, et al. Vitamin B
12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008; 51:29–38. PMID:
17851649.
39. Soliman ML, Rosenberger TA. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol Cell Biochem. 2011; 352:173–180. PMID:
21359531.
40. Dashwood RH, Ho E. Dietary histone deacetylase inhibitors: from cells to mice to man. Semin Cancer Biol. 2007; 17:363–369. PMID:
17555985.
41. Kulkarni A, Dangat K, Kale A, Sable P, Chavan-Gautam P, Joshi S. Effects of altered maternal folic acid, vitamin B12 and docosahexaenoic acid on placental global DNA methylation patterns in Wistar rats. PLoS One. 2011; 6:e17706. PMID:
21423696.
42. Ceccarelli V, Racanicchi S, Martelli MP, Nocentini G, Fettucciari K, Riccardi C, Marconi P, Di Nardo P, Grignani F, Binaglia L, et al. Eicosapentaenoic acid demethylates a single CpG that mediates expression of tumor suppressor CCAAT/enhancer-binding protein delta in U937 leukemia cells. J Biol Chem. 2011; 286:27092–27102. PMID:
21659508.
43. Moreira JC, Dal-Pizzol F, Rocha AB, Klamt F, Ribeiro NC, Ferreira CJ, Bernard EA. Retinol-induced changes in the phosphorylation levels of histones and high mobility group proteins from Sertoli cells. Braz J Med Biol Res. 2000; 33:287–293. PMID:
10719379.
44. Chung TL, Brena RM, Kolle G, Grimmond SM, Berman BP, Laird PW, Pera MF, Wolvetang EJ. Vitamin C promotes widespread yet specific DNA demethylation of the epigenome in human embryonic stem cells. Stem Cells. 2010; 28:1848–1855. PMID:
20687155.
45. McKay JA, Mathers JC. Diet induced epigenetic changes and their implications for health. Acta Physiol (Oxf). 2011; 202:103–118. PMID:
21401888.
46. Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2012; 13:97–109. PMID:
22215131.
47. Zhang N. Epigenetic modulation of DNA methylation by nutrition and its mechanisms in animals. Anim Nutr. 2015; 1:144–151. PMID:
29767106.
48. Kanfi Y, Peshti V, Gozlan YM, Rathaus M, Gil R, Cohen HY. Regulation of SIRT1 protein levels by nutrient availability. FEBS Lett. 2008; 582:2417–2423. PMID:
18544345.
49. Ribarič S. Diet and aging. Oxid Med Cell Longev. 2012; 2012:741468. PMID:
22928085.
50. Donohoe DR, Bultman SJ. Metaboloepigenetics: interrelationships between energy metabolism and epigenetic control of gene expression. J Cell Physiol. 2012; 227:3169–3177. PMID:
22261928.
51. DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008; 7:11–20. PMID:
18177721.
52. Pan M, Reid MA, Lowman XH, Kulkarni RP, Tran TQ, Liu X, Yang Y, Hernandez-Davies JE, Rosales KK, Li H, et al. Regional glutamine deficiency in tumours promotes dedifferentiation through inhibition of histone demethylation. Nat Cell Biol. 2016; 18:1090–1101. PMID:
27617932.
53. Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008; 14:72–81. PMID:
18218340.
54. Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008; 49:1068–1076. PMID:
18227531.
55. Yamazaki Y, Kakizaki S, Takizawa D, Ichikawa T, Sato K, Takagi H, Mori M. Interstrain differences in susceptibility to non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2008; 23:276–282. PMID:
17868334.
56. Larter CZ, Yeh MM, Haigh WG, Williams J, Brown S, Bell-Anderson KS, Lee SP, Farrell GC. Hepatic free fatty acids accumulate in experimental steatohepatitis: role of adaptive pathways. J Hepatol. 2008; 48:638–647. PMID:
18280001.
57. Pogribny IP, Tryndyak VP, Bagnyukova TV, Melnyk S, Montgomery B, Ross SA, Latendresse JR, Rusyn I, Beland FA. Hepatic epigenetic phenotype predetermines individual susceptibility to hepatic steatosis in mice fed a lipogenic methyl-deficient diet. J Hepatol. 2009; 51:176–186. PMID:
19450891.
58. Boffa LC, Vidali G, Mann RS, Allfrey VG. Suppression of histone deacetylation
in vivo and
in vitro by sodium butyrate. J Biol Chem. 1978; 253:3364–3366. PMID:
649576.
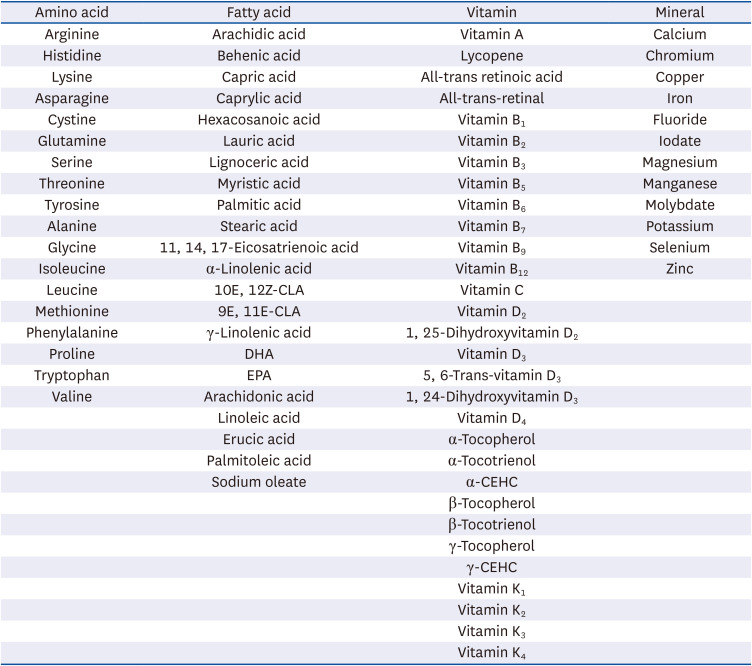
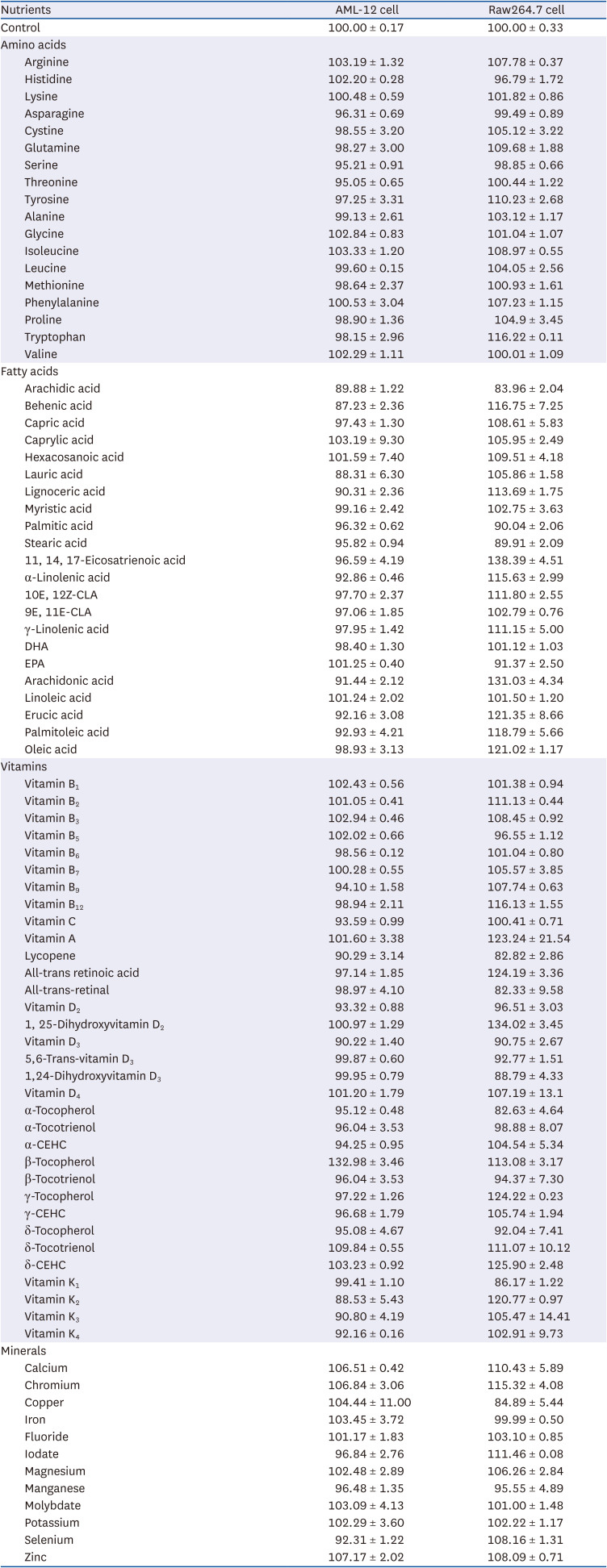
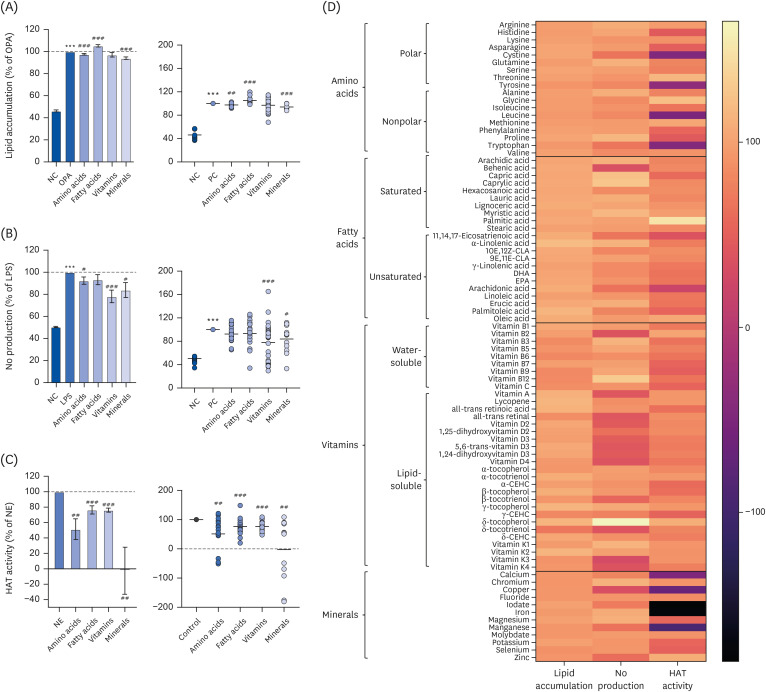
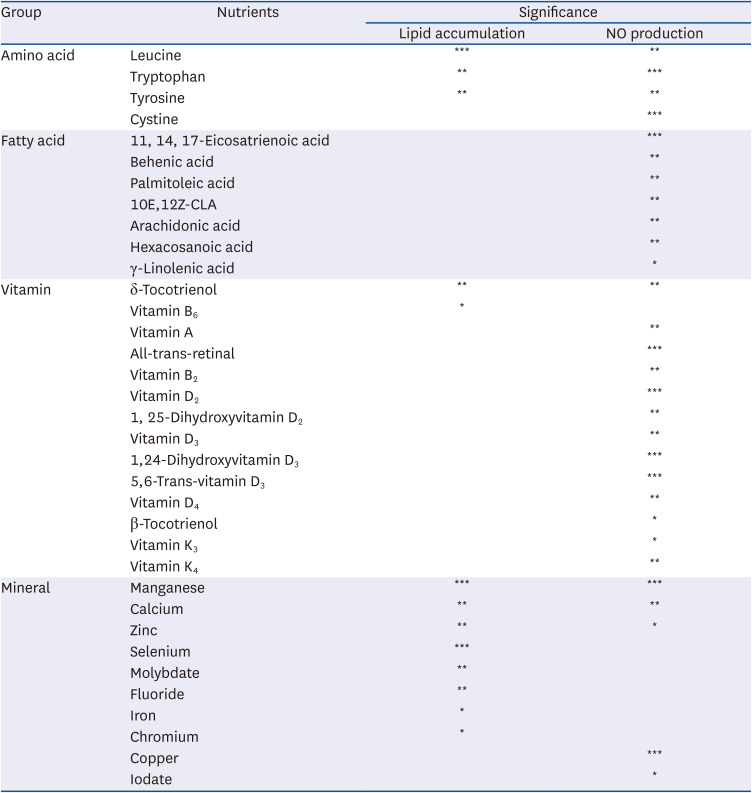




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download