A 64-year-old woman presented with dyspnea for a year. She was diagnosed with systemic sclerosis (SSc) in 2014 and no pulmonary and cardiovascular complications were observed until 2020. The dyspnea onset in early 2021 led her to a cardiologist and transthoracic echocardiography showed biventricular dilatation and a left ventricular ejection fraction of 20%. Cardiac magnetic resonance imaging (CMRI) showed extensive late gadolinium enhancement (LGE) subendocardium of left ventricular walls, which suggested multi-vessel coronary artery occlusion (Figure 1A-D). However, there was no significant epicardial coronary artery obstruction on coronary angiography (Figure 1E and F). One year later, she was admitted for aggravating dyspnea despite guideline-directed medical therapy and became dobutamine dependent state. The right heart catheterization showed biventricular systolic dysfunction, but no pulmonary hypertension was noted (pulmonary artery pressure, systolic/diastolic/mean: 27/16/19 mmHg, pulmonary vascular resistance: 1.8 wood unit). She received a heart transplant on day 53 after hospitalization. Pathologic examination of the explanted heart revealed severe endocardial fibrosis and multifocal patch fibrosis of the myocardium in both ventricles (Figure 2). Furthermore, severe microvascular disease was noted. Most intracardiac arteries including atrioventricular nodal artery and arterioles showed fibromuscular dysplastic changes with luminal narrowing (Figure 3) consistent with ischemic pattern LGE in CMRI. Similar to the previous report,1) the principal pathogenic process in this SSc case was the extensive microvascular occlusion, leading to distinguished ischemic cardiomyopathy from atherosclerotic ischemia.
Written informed consent was obtained from the patient.
Notes
Funding: This study was supported by a 2022 research grant from Pusan National University Yangsan Hospital.
References
1. Kawano H, Kudo T, Umeda M, Futakuchi M, Sueyoshi E, Maemura K. Myocardial damage and microvasculopathy in a patient with systemic sclerosis. Circ J. 2021; 85:224. PMID: 33342919.

Figure 1
CMRI and coronary angiography of the systemic sclerosis heart. (A-D) Late gadolinium-enhancement CMRI of the explanted heart. An extensive subendocardial enhancement of the left ventricle and multifocal enhancement in the right ventricle was noted (white arrows). (E, F) Coronary angiography of the heart. (E) Left coronary angiography and (F) right coronary angiography revealed no significant epicardial coronary artery stenosis.
CMRI = cardiac magnetic resonance imaging.
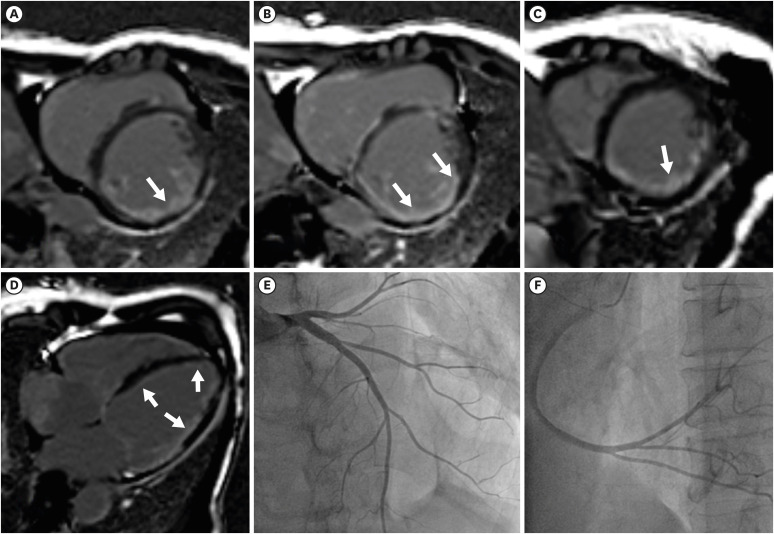
Figure 2
Pathologic findings. (A) Gross appearance of the explanted heart. Cross-sections of the entire heart specimen showed ventricular dilation and prominent endocardial fibrosis in both ventricles (arrow, whitish tissues). (B, C) Low magnification of the inferior wall of the left ventricle showed wall thinning, endocardial thickening with fibrosis, and patchy myocardial fibrosis. (B) Hematoxylin and eosin stain; (C) Masson’s trichrome stain.
Endo = endocardium; Epi = epicardium; Myo = myocardium.
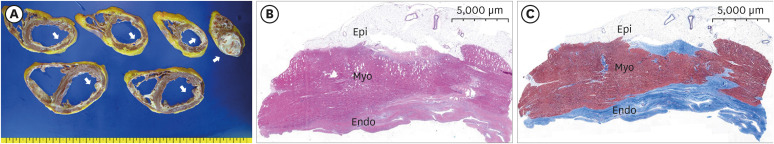
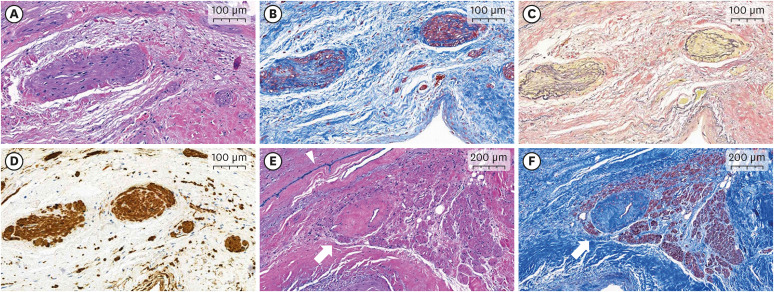
Figure 3
Microvascular alterations in pathologic specimens of the explanted systemic sclerosis heart. (A-D) Microscopic examination reveals fibromuscular dysplastic changes in the small vessels of the subendocardial myocardium. These micro-vasculatures show luminal narrowing due to fibrosis and smooth muscle hyperplasia in the tunica intima and media. Fragmentation or duplication of the internal elastic lamina is also noted. (A) Hematoxylin and eosin stain, ×100; (B) Masson’s trichrome stain, ×100; (C) Verhoeff’s elastic stain, ×100; (D) Immunohistochemical stain for smooth muscle actin, ×100. (E, F) An atrioventricular nodal artery shows luminal narrowing and vascular wall fibrosis. (E) Hematoxylin and eosin stain, ×50; (F) Masson’s trichrome stain, ×50. (E, F) White arrows indicate a nodal artery and (E) blue ink indicates the right side of the interventricular septum (arrowhead).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download