1. McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014; 2:843–851. PMID:
24731668.

2. Kong MG, Jang SY, Jang J, et al. Impact of diabetes mellitus on mortality in patients with acute heart failure: a prospective cohort study. Cardiovasc Diabetol. 2020; 19:49. PMID:
32359358.

3. Jang SY, Jang J, Yang DH, et al. Impact of insulin therapy on the mortality of acute heart failure patients with diabetes mellitus. Cardiovasc Diabetol. 2021; 20:180. PMID:
34496864.

4. Sharma A, Pagidipati NJ, Califf RM, et al. Impact of regulatory guidance on evaluating cardiovascular risk of new glucose-lowering therapies to treat type 2 diabetes mellitus: lessons learned and future directions. Circulation. 2020; 141:843–862. PMID:
31992065.

5. Parng C, Seng WL, Semino C, McGrath P. Zebrafish: a preclinical model for drug screening. Assay Drug Dev Technol. 2002; 1:41–48. PMID:
15090155.
6. Grunwald DJ, Streisinger G. Induction of recessive lethal and specific locus mutations in the zebrafish with ethyl nitrosourea. Genet Res. 1992; 59:103–116. PMID:
1628817.

7. Jurczyk A, Roy N, Bajwa R, et al. Dynamic glucoregulation and mammalian-like responses to metabolic and developmental disruption in zebrafish. Gen Comp Endocrinol. 2011; 170:334–345. PMID:
20965191.
8. Howe K, Clark MD, Torroja CF, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013; 496:498–503. PMID:
23594743.
9. Missinato MA, Zuppo DA, Watkins SC, Bruchez MP, Tsang M. Zebrafish heart regenerates after chemoptogenetic cardiomyocyte depletion. Dev Dyn. 2021; 250:986–1000. PMID:
33501711.
10. Huang CJ, Tu CT, Hsiao CD, Hsieh FJ, Tsai HJ. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev Dyn. 2003; 228:30–40. PMID:
12950077.

11. Quan H, Oh GC, Seok SH, Lee HY. Fimasartan, an angiotensin II receptor antagonist, ameliorates an in vivo zebrafish model of heart failure. Korean J Intern Med. 2020; 35:1400–1410. PMID:
32164398.

12. Gu G, Na Y, Chung H, Seok SH, Lee HY. Zebrafish Larvae Model of Dilated Cardiomyopathy Induced by Terfenadine. Korean Circ J. 2017; 47:960–969. PMID:
29035434.
13. Members of the Panel on Euthanasia. AVMA guidelines for the euthanasia of animals: 2020 edition. Schaumburg (IL): American Veterinary Medical Association;2020.
14. diIorio PJ, Moss JB, Sbrogna JL, Karlstrom RO, Moss LG. Sonic hedgehog is required early in pancreatic islet development. Dev Biol. 2002; 244:75–84. PMID:
11900460.
15. Andersson O, Adams BA, Yoo D, et al. Adenosine signaling promotes regeneration of pancreatic β cells in vivo. Cell Metab. 2012; 15:885–894. PMID:
22608007.
16. Lee Y, Yang J. Development of a zebrafish screening model for diabetic retinopathy induced by hyperglycemia: Reproducibility verification in animal model. Biomed Pharmacother. 2021; 135:111201. PMID:
33421732.
17. Singh A, Castillo HA, Brown J, Kaslin J, Dwyer KM, Gibert Y. High glucose levels affect retinal patterning during zebrafish embryogenesis. Sci Rep. 2019; 9:4121. PMID:
30858575.
18. Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001; 50:537–546. PMID:
11829314.
19. Huang CC, Chen PC, Huang CW, Yu J. Aristolochic acid induces heart failure in zebrafish embryos that is mediated by inflammation. Toxicol Sci. 2007; 100:486–494. PMID:
17823451.
20. Matrone G, Taylor JM, Wilson KS, et al. Laser-targeted ablation of the zebrafish embryonic ventricle: a novel model of cardiac injury and repair. Int J Cardiol. 2013; 168:3913–3919. PMID:
23871347.

21. Jiménez-Amilburu V, Jong-Raadsen S, Bakkers J, Spaink HP, Marín-Juez R. GLUT12 deficiency during early development results in heart failure and a diabetic phenotype in zebrafish. J Endocrinol. 2015; 224:1–15. PMID:
25326603.

22. Kronlage M, Dewenter M, Grosso J, et al. O-GlcNAcylation of histone deacetylase 4 protects the diabetic heart from failure. Circulation. 2019; 140:580–594. PMID:
31195810.
23. Jeon YW, Kim HC. Factors associated with awareness, treatment, and control rate of hypertension among Korean young adults aged 30-49 years. Korean Circ J. 2020; 50:1077–1091. PMID:
32975054.

24. Zang L, Shimada Y, Nishimura N. Development of a novel zebrafish model for type 2 diabetes mellitus. Sci Rep. 2017; 7:1461. PMID:
28469250.

25. Zhang M, Lv XY, Li J, Xu ZG, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2008; 2008:704045. PMID:
19132099.

26. Tanaka S, Hayashi T, Toyoda T, et al. High-fat diet impairs the effects of a single bout of endurance exercise on glucose transport and insulin sensitivity in rat skeletal muscle. Metabolism. 2007; 56:1719–1728. PMID:
17998027.
27. Kolk MV, Meyberg D, Deuse T, et al. LAD-ligation: a murine model of myocardial infarction. J Vis Exp. 2009; (32):1438. PMID:
19829290.

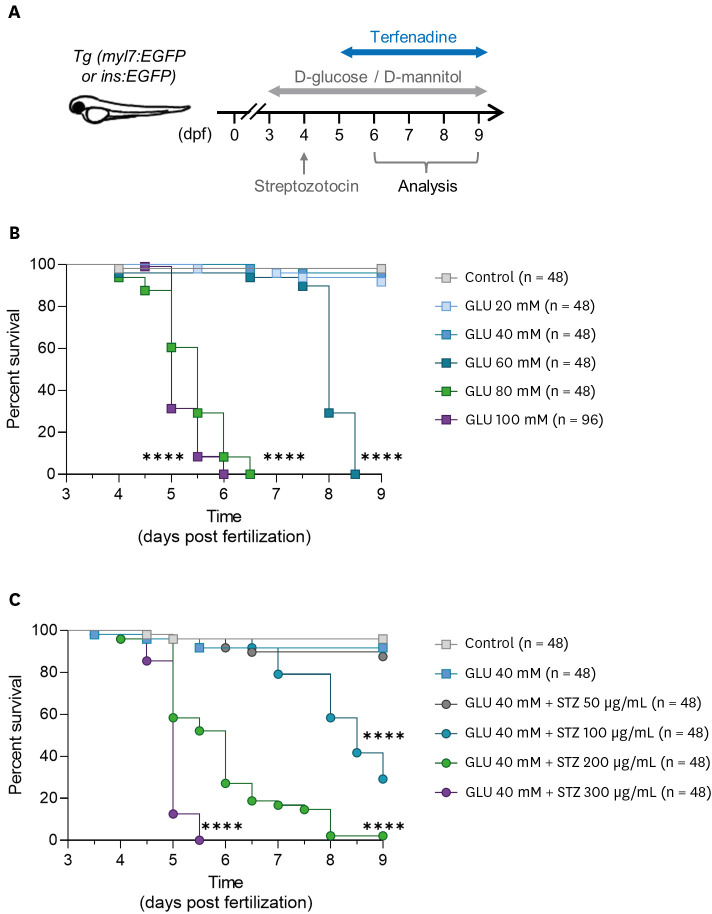
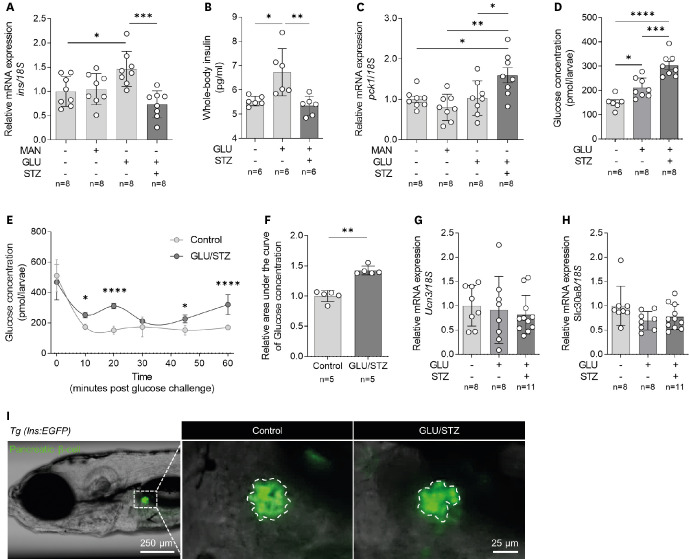
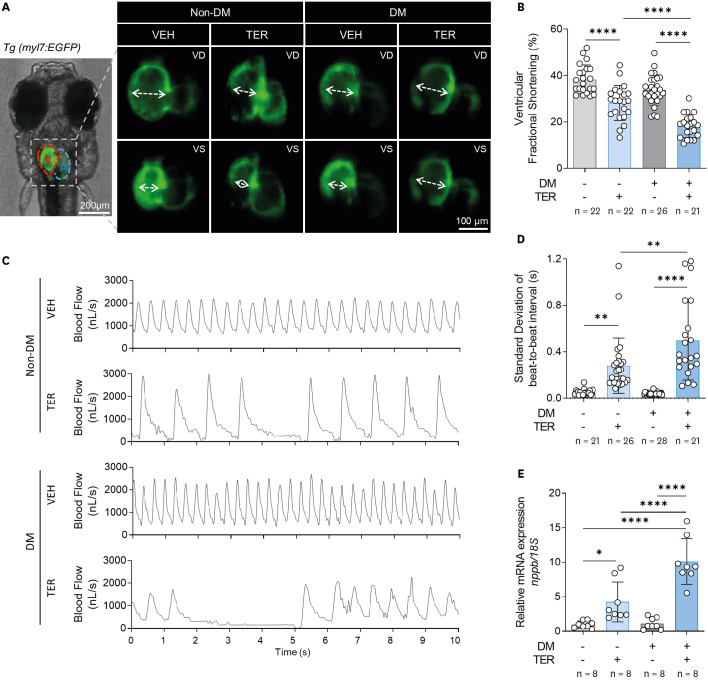
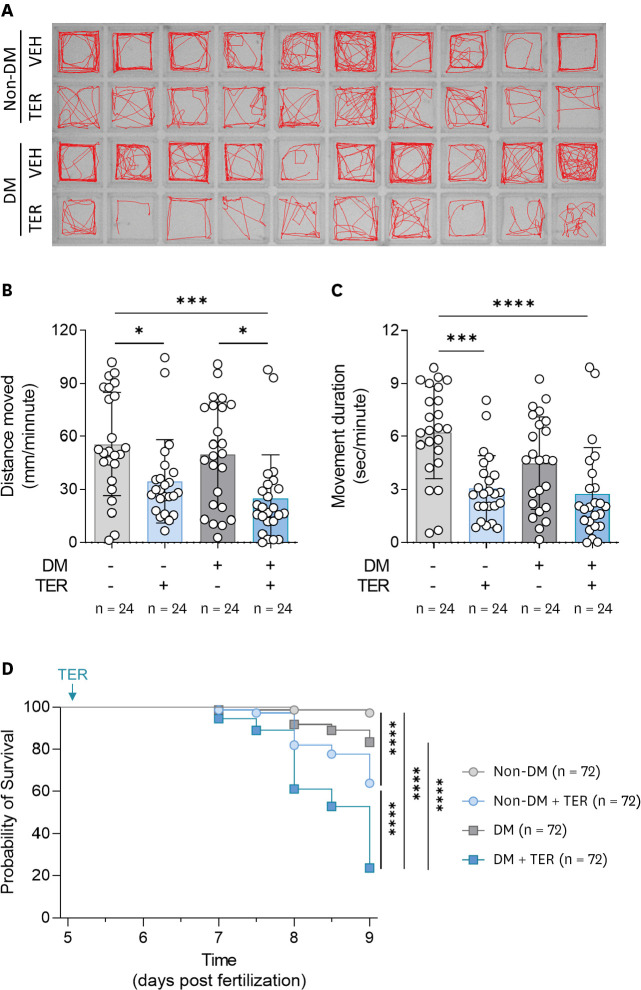




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download