INTRODUCTION
Increased esthetic expectations together with the need for functional balance of the jaws make orthodontic treatment of skeletal deformities a demanding procedure. Class III malocclusion is clinically characterized by a retrusive upper face and a protrusive lower face, thus creating a concave profile. Two-thirds of skeletal Class III malocclusions are caused by maxillary retrusion or a combination of maxillary retrusion and mandibular prognathism.
1,2 Given the frequency of maxillary retrusion, maxillary protraction has become a major treatment approach for the early correction of Class III malocclusion.
2,3 Traditional maxillary protraction with a facemask (FM) involves both skeletal and dentoalveolar effects, including the forward movement of the maxilla, counter-clockwise rotation of the palatal plane, clockwise rotation of the mandible, labial tipping of the maxillary incisors, and lingual tipping of the mandibular incisors.
4 Although the primary purpose of maxillary protraction therapy is to advance the maxilla, the maxillary forward movement after 6–12 months of treatment is limited to 2–3 mm.
3 Furthermore, the unwanted dentoalveolar effects, relapse due to late mandibular growth, and inability to advance the maxilla after the adolescent growth spurt have led orthodontists to search for other treatment modalities. Bone-anchored maxillary protraction (BAMP) allows maxillary advancement and mandibular growth restriction with minimal dentoalveolar effects.
5 Moreover, after the introduction of distraction osteogenesis of the maxilla, surgically assisted maxillary protraction has become an alternative protocol in severe cases or even after the adolescent growth spurt, with maxillary advancement of 3–12 mm achievable in a shorter amount of time than with the conventional protocols.
6-8
Previous studies have primarily examined the skeletal and dental effects of different treatment protocols. Although the primary aim of using surgical assistance (modified Le Fort I osteotomy) before maxillary protraction is to increase the amount of maxillary advancement in severe cases or after the adolescent growth spurt, other potential effects of this approach, such as changes in the soft tissue and airway should also be taken into consideration. Recent studies have shown that several factors can contribute to obstructive sleep apnea (OSA) in pediatric patients, including tonsillar or adenoid hypertrophy, maxillary or mandibular deficiency, obesity, and craniofacial anomalies.
9 Thus, in some cases, maxillary protraction treatment can yield improvements in OSA in addition to showing favorable skeletal and dental effects.
Numerous studies have investigated the effects of maxillary protraction on the pharyngeal airway; however, the results of these studies are conflicting.
10-18 While some authors have found significant changes in both oropharyngeal and nasopharyngeal airway dimensions,
13,14 others found changes only in nasopharyngeal airway dimensions.
15-17 Furthermore, some studies reported no significant differences at all.
10-12 However, comparisons of the results of such studies should be performed while considering methodological differences, sample differences, and treatment times. In most of the abovementioned studies, FM was used as a protraction device for longer periods in different age groups, and the average sella to nasion angle (SNA) changes were between 1.3° and 2.9°.
Based on the knowledge that surgically assisted maxillary protraction increases the amount of maxillary protraction within a shorter period than that of the conventional methods, one can assume that this treatment protocol would have a beneficial effect on the pharyngeal airway. To the best of our knowledge, only one study has evaluated the effects of surgically assisted maxillary protraction on airway dimensions, and it found statistically significant changes only in nasopharyngeal airway dimensions.
18 However, the authors of that study also used FM. The present study is the first to two-dimensionally (2D) evaluate changes in pharyngeal airway dimensions during the control period after surgically assisted maxillary protraction with skeletal anchorage and Class III elastics, and during follow-up.
RESULTS
All the skeletal and airway measurements were repeated after 10 days by the same examiner, and the intraclass correlation coefficient of all the parameters ranged from 0.8 to 0.9, showing a high level of agreement. No statistically significant changes were observed in the skeletal and airway parameters during the control period (T1–T0) (
p > 0.05,
Table 3).
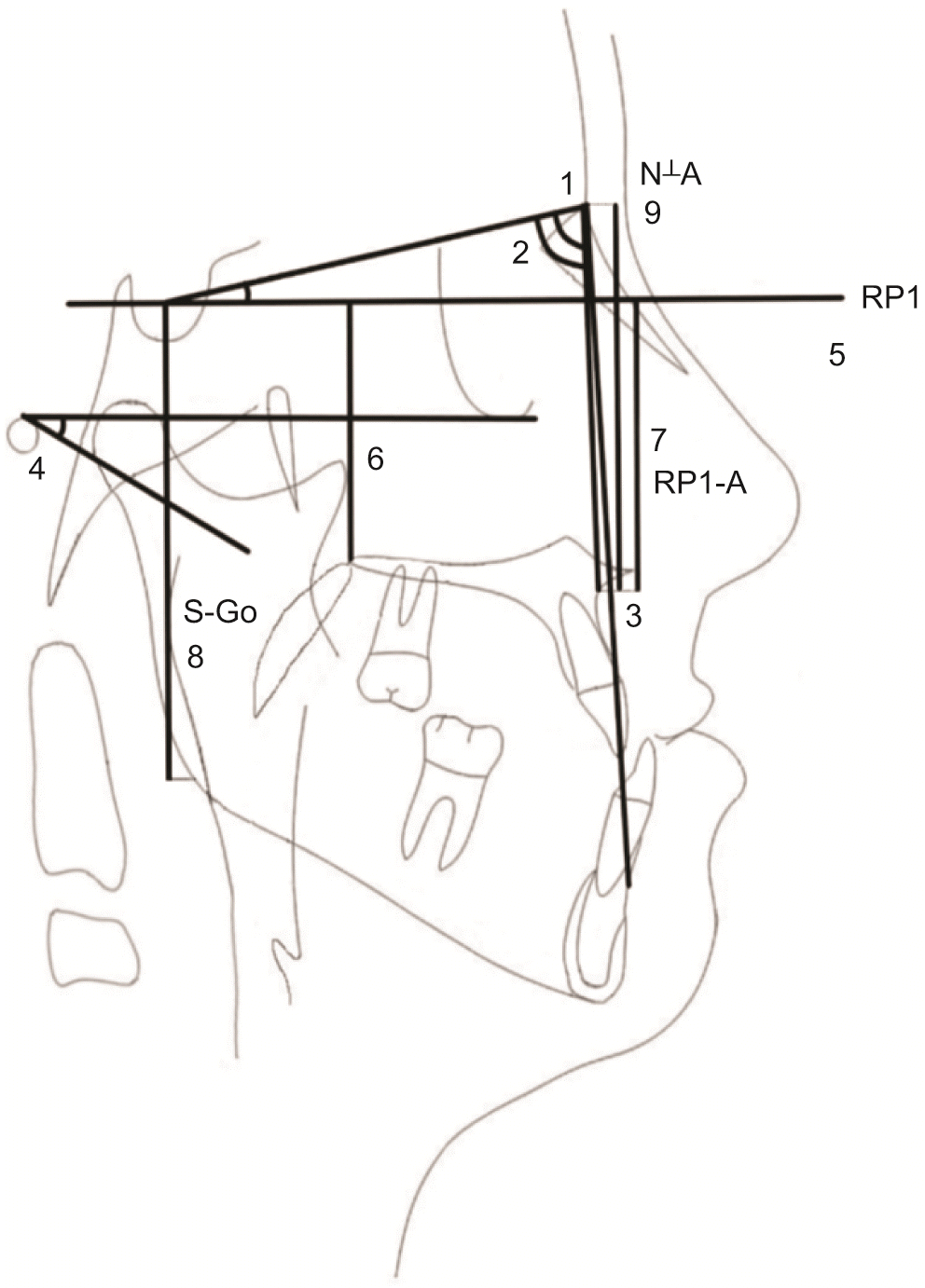
During the maxillary protraction period (T2–T1), although significant increases were observed in SNA, N⊥A, ANB, and Frankfort plane to mandibular plane angle (FMA) (2.9° ± 1.1°, 3.7 ± 1.4 mm, 3.9° ± 2.0°, 1.2° ± 1.0°, respectively,
p < 0.05), the decrease in the SNB angle and horizontal reference plane to A point (RP1-A) distance from T1 to T2 (–1.0° ± 1.5°, –0.1 ± 1.1 mm, respectively,
p > 0.05) was not statistically significant (
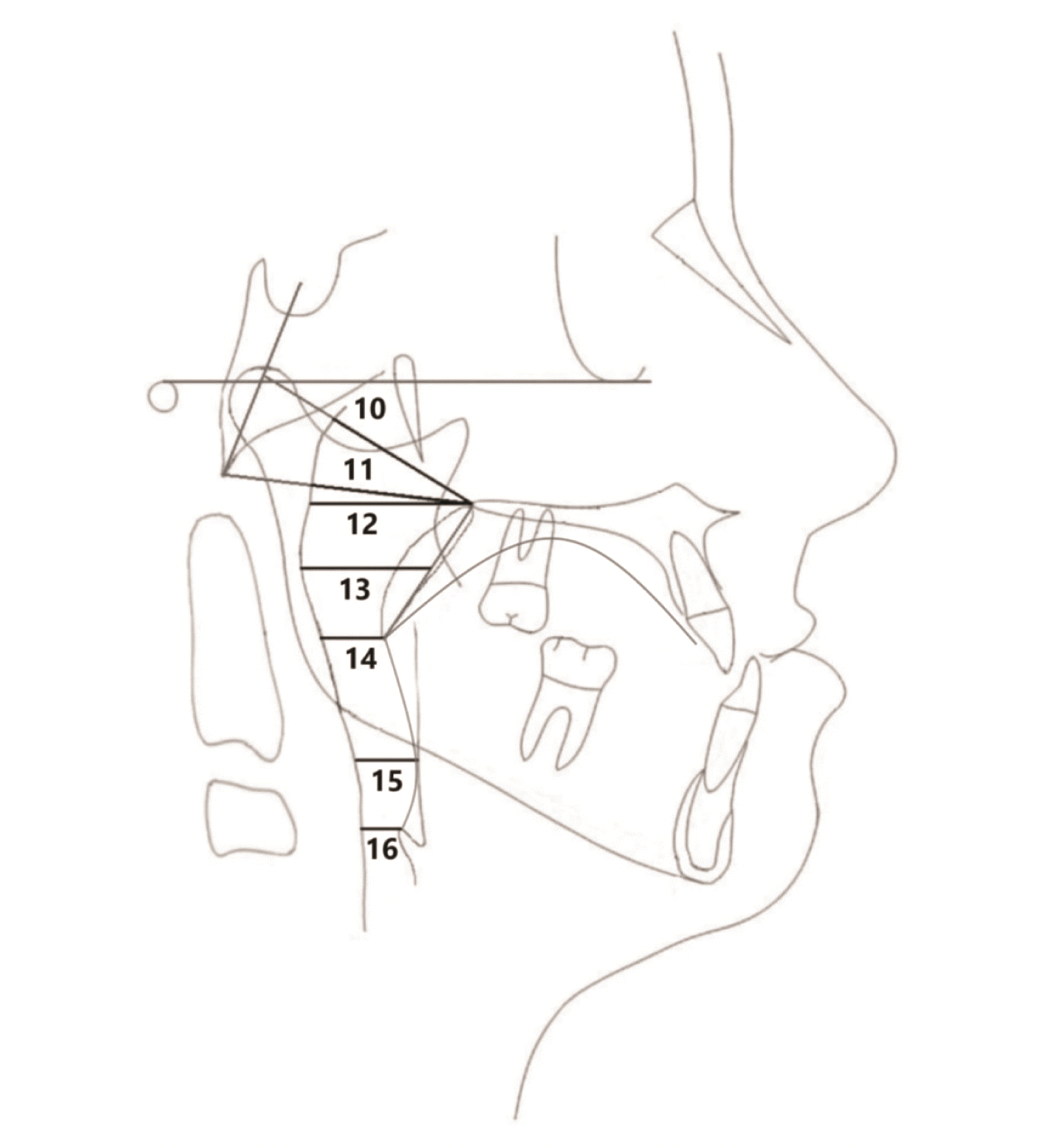
Table 3). Among the airway parameters, although the sagittal airway dimensions increased (adenoid tissue 1 to posterior nasal spine [Ad1-PNS]: 0.2 ± 3.1 mm; Adenoid tissue 2 to posterior nasal spine [Ad2-PNS]: 1.1 ± 2.2 mm; posterior airway space [PAS]: 0.7 ± 2.7 mm; superoposterior airway space [SPAS]: 0.2 ± 1.7 mm,
p > 0.05) and decreased (middle airway space [MAS]: –0.4 ± 2.3 mm; inferior airway space [IAS]: –0.1 ± 3.4 mm; epiglottic airway space [EAS]: –0.8 ± 2.2 mm,
p > 0.05) during the maxillary protraction period, the changes were not statistically significant (
Table 3,
Figure 4). During the follow-up period (T3–T2), none of the airway parameters showed statistically significant changes (Ad1-PNS: –0.9 ± 6.3 mm; Ad2-PNS: 1.3 ± 3.1 mm; PAS: –0.7 ± 3.5 mm; SPAS: –0.6 ± 2.0 mm; MAS: 0.5 ± 2.1 mm; IAS: –0.7 ± 3.2 mm; EAS: 0.4 ± 1.1 mm,
p > 0.05). Among the skeletal parameters, only the S-Go distance showed a significant increase (4.0 ± 2.5 mm,
p < 0.05) (
Table 3).
When the entire period from control to follow-up was evaluated (T3–T0), only Ad2-PNS increased significantly (3.0 ± 3.7 mm,
p < 0.05) among the airway parameters (
Table 3). Moreover, significant increases were seen in RP1-PNS, SNA, N⊥A, ANB, and S-Go (1.7 ± 1.7 mm, 3.4° ± 1.5°, 3.8 ± 2.2 mm, 3.8° ± 1.3°, and 5.1 ± 3.0 mm, respectively,
p < 0.05) (
Table 3).
DISCUSSION
Maxillary protraction with FM or orthognathic surgery are the most favored treatment protocols for Class III malocclusion. Besides the limited maxillary advancement (2–3 mm in 6–12 months) and need for treatment at young ages with FM,
3 patients have to wait until growth is completed for any orthognathic surgery. However, considering the psychological problems caused by decreased facial esthetics, treatment of severe cases involving young adolescents or those after the growth spurt is very important, instead of waiting until adulthood. Orthognathic surgery at an early age
21 and surgical assistance with modified Le Fort I osteotomy or skeletal anchorage for maxillary protraction
6,8,22,23 in these groups of patients have been reported in the literature. Among these methods, treatment with orthognathic surgery at early ages may inhibit the further growth of the maxilla due to the use of miniplates and miniscrews for fixation and the extended osteotomy lines, and the continued mandibular growth may cause recurrence of the Class III malocclusion.
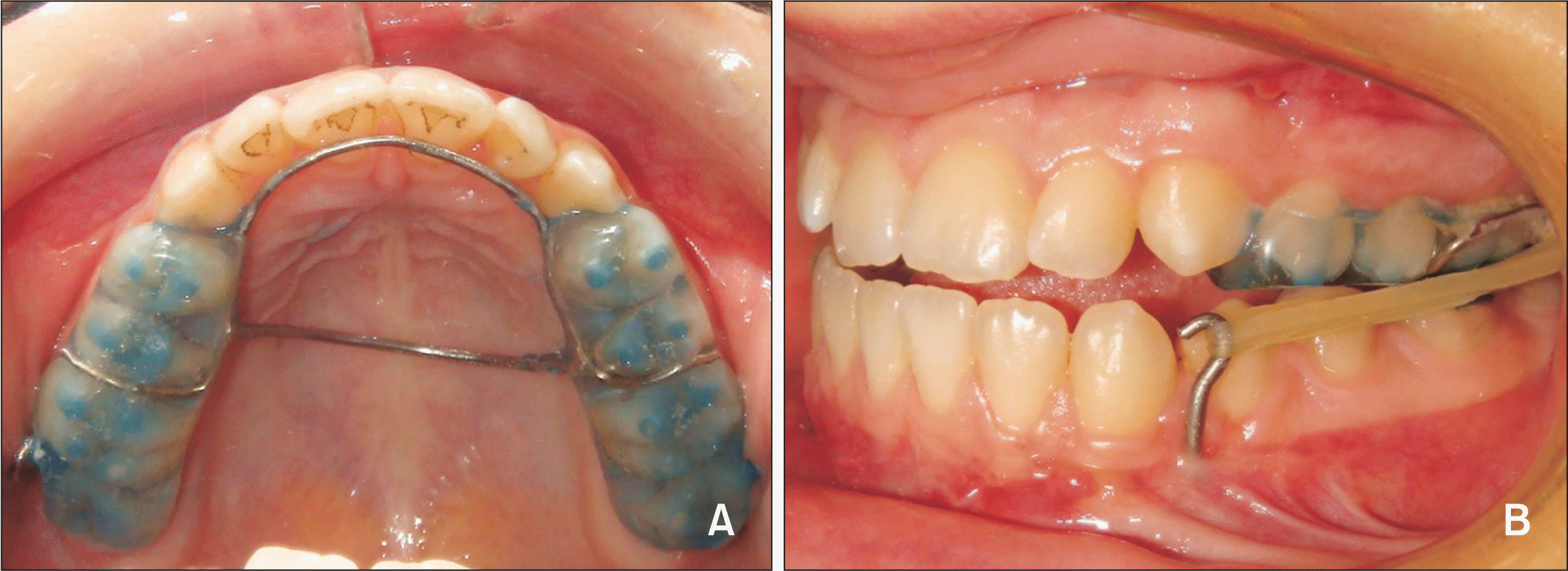
21 No down fracture and no rigid fixation are performed, and the nasal septum and nasal walls are kept intact in the surgical assistance (with modified Le Fort I osteotomy) for maxillary protraction.
7 Therefore, anteroposterior maxillary growth can be expected in response to future mandibular growth.
21
The amount of maxillary advancement was around 2.3–4.8 mm in 6–12 months with skeletal anchorage,
22,23 and 3.5–12 mm in 1–5 months with surgical assistance for maxillary protraction.
6-8 This leads to a critical question, “Do such protraction methods assisted by either surgical procedure or skeletal anchorage have any effects on airway dimensions?” Studies have reported conflicting results about the relationship between maxillary protraction and changes in airway dimensions.
10-18 Hiyama et al.
10 showed that greater forward maxillary growth was associated with a greater increase in the upper airway dimensions. Since surgically assisted maxillary protraction with skeletal anchorage would produce more maxillary anterior movement in a shorter time than- that of the conventional maxillary protraction, we expected to find more significant improvements in pharyngeal airway dimensions in the present study.
It is crucial to measure the actual treatment outcome while excluding the individuals' expected growth. While some studies have reported their results without a control group, some used Class I or Class III control groups. Since the present study is retrospective in nature, the control records of the same individuals that were taken 5.5 months prior to maxillary protraction were used to differentiate the expected growth of the participants. That control duration was determined according to the predicted time for the maxillary protraction period. Therefore, each individual served as a control group for themselves, and no additional control group was created for ethical reasons.
Most of the published studies evaluated changes in the pharyngeal airway using lateral cephalograms. Although three-dimensional (3D) evaluation of complex anatomy, such as the airway, provides more detailed information, the use of cephalograms has also been shown to be adequate and reliable for measuring sagittal airway dimensions.
13-16 Owing to the retrospective design of the present study, lateral cephalometric radiographs of the participants obtained from an archive were used to measure airway changes.
In the present study, a limited number of skeletal variables that were considered to be associated with airway changes were evaluated to avoid distraction and focus on the pharyngeal airway. During the control period (T1–T0), no statistically significant changes were observed in any of the skeletal or airway parameters. However, during protraction (T2–T1), the maxilla moved forward significantly, resulting in increases in the SNA, N⊥A, ANB, and FMA angles (2.9° ± 1.1°, 3.7 ± 1.4 mm, 3.9° ± 2.0°, 1.2° ± 1.0°, respectively), while the SNB, RP1-A, RP1-PNS, and S-Go did not change significantly. The changes in the skeletal parameters were compatible with previously published findings in which maxillary protraction with surgical assistance and skeletal anchorage showed significant forward movement in the maxilla.
6,8,22,23 No significant changes were observed in the pharyngeal airway dimensions (Ad1-PNS: 0.2 ± 3.1 mm; Ad2-PNS: 1.1 ± 2.2 mm; PAS: 0.7 ± 2.7 mm; SPAS: 0.2 ± 1.7 mm; MAS: –0.4 ± 2.3 mm; IAS: –0.1 ± 3.4 mm; EAS: –0.8 ± 2.2 mm) during the protraction period. The findings of the present study regarding the pharyngeal airway are consistent with those reported by Hiyama et al.
10 (SPAS: 0.5 ± 3.2 mm; MAS: –0.3 ± 4.5 mm; IAS: –0.2 ± 3.9 mm), Baccetti et al.
11 (Ad1-PNS: 2.8 ± 3.2 mm; Ad2-PNS: 3.2 ± 2.8 mm; upper pharynx: 2.0 ± 2.7 mm; lower pharynx: 0.0 ± 3.5 mm), and Mucedero et al.
12 (Ad1-PNS: 2.1 ± 3.9 mm; Ad2-PNS: 1.2 ± 2.2 mm; upper pharynx: 1.0 ± 3.8 mm; lower pharynx: 0.7 ± 3.1 mm) who reported statistically insignificant results; however, they contradict the studies that reported significant increases in the dimensions of the nasopharynx and/or oropharynx.
13-18,24,25 The absence of the expected changes in pharyngeal airway dimensions due to greater maxillary advancement in the present study might be attributable to the physiological growth of lymphoid tissue.
26 According to Taylor et al.,
27 posterior pharyngeal wall changes occur at a greater rate between 6 to 9 and 12 to 15 years of age. In the present study, while the average age of the patients when they underwent maxillary protraction was 13.4 ± 1.2 years, the patients in most previous studies were 9–12 years old. Thus, the patients in the present study group were in the age range where reductions in pharyngeal airway dimensions are expected due to normal growth, which might neutralize the effects of maxillary protraction. Only Cakirer et al.
18 evaluated the effects of surgically assisted maxillary protraction and found significant increases in nasopharyngeal airway dimensions (Ad2-PNS: 2.4 ± 2.5 mm); however, the age of the patients, the type and duration of protraction, and the amount of maxillary movement differed in their study, which might be the reason for the differences in relation to the present study.
Furthermore, although Kilinç et al.
13 found significant increases in both nasopharyngeal (Ad1-PNS: 4.6 ± 5.3 mm; Ad2-PNS: 5.6 ± 1.8 mm) and oropharyngeal (1.4 ± 4.3 mm) airway dimensions, they also stated that their findings were not statistically significant due to individual variations when compared to a control group. Ceylan and Oktay
28 reported that the oropharyngeal airway dimension decreases with an increase in the ANB angle. Akcam et al.
29 reported that the clockwise rotation of the mandible decreases the airway space. In contrast, Hiyama et al.
10 found no significant relationship between SNB and changes in airway dimensions in their multiple-regression analysis. In the present study, while SNB did not change significantly during protraction (–1.0° ± 1.5°), the significant increase in the FMA angle (1.2° ± 1.0°) indicated a slight clockwise rotation of the mandible. However, this slight rotation did not appear to cause a significant change in oropharyngeal airway dimensions, which was consistent with the findings of other studies.
10,15,24
No significant changes were observed in SNA or ANB angles during the follow-up period (T2–T3), indicating that maxillary protraction was maintained after treatment, as reported elsewhere.
11,16 In contrast to our results, Nevzatoğlu and Küçükkeleş
6 reported that after almost 6 years of follow-up, the maxillary sagittal changes achieved with surgically assisted maxillary protraction were lost, while the changes achieved with conventional FM were stable. Significant increases in S-Go distances (4.0 ± 2.5 mm) were seen during follow-up in the present study. This continued significant growth in the posterior facial height (S-Go) may have caused clockwise rotation and anterior rotation of the mandible, resulting in an insignificant decrease in the FMA angle.
7 Additionally, the small change in RP1-A may be an indication that the vertical growth of the maxilla was not inhibited by the modified Le Fort I osteotomy, which did not include down fracture or rigid fixation. Furthermore, since the nasal walls and nasal septum were not included in the protocol, no anteroposterior growth restriction was expected.
21 None of the changes in pharyngeal airway measurements (Ad1-PNS: –0.9 ± 6.3 mm; Ad2-PNS: 1.3 ± 3.1 mm; PAS: –0.7 ± 3.5 mm; SPAS: –0.6 ± 2.0 mm; MAS: 0.5 ± 2.1 mm; IAS: –0.7 ± 3.2 mm; and EAS: 0.4 ± 1.1 mm) were significant during the follow-up period in the present study. The long-term results of the changes in upper airway dimensions are also still controversial. Kaygisiz et al.
16 reported that any significant changes in nasopharyngeal airway dimensions (Ad1-PNS: 1.4 ± 2.5 mm; Ad2-PNS: 2.9 ± 3.0 mm) remained stable in the long-term. However, Baccetti et al.
11 and Hwang et al.
25 showed insignificant results during the follow-up period, which is consistent with the findings of the present study.
The treatment protocol in the present study did not include maxillary expansion since the participants did not require significant maxillary expansion after maxillary protraction. Inclusion of maxillary expansion in this treatment protocol may have raised the question of whether a significant change in the airway could be observed. However, Adobes Martin et al.
30 reported that maxillary protraction alone may cause an increase in the pharyngeal airway. They also added that maxillary expansion had no effect on sagittal widths in comparison with controls and since it is related to the transversal dimensions of the malocclusion, the effect on the transverse dimension cannot be quantified on a 2D image.
One of the limitations of this retrospective study was the use of a 2D imaging technique for the assessment of a 3D structure such as the pharyngeal airway. However, lateral cephalometric radiographs are frequently used to evaluate pharyngeal structures.
10-18 Although 3D measurements are expected to provide more precise information on the pharyngeal airway, the results of 3D studies in the literature regarding the airway are also contradictory. Nguyen et al.
31 found significant increments in upper airway dimensions following the BAMP protocol in their 3D study; however, when comparing their results with a well-matched control group, the results were similar. Furthermore, some studies have reported no significant changes in airway volumes.
32,33 Other limitations of the present study were the lack of an untreated control group and the retrospective nature of the study. The fact that this treatment protocol can be used in some “selected” Class III cases might also be considered a limitation. Therefore, case selection is of utmost importance when this protocol is used.
Future prospective 3D studies with a larger study sample and a consensus for the demarcation of the pharyngeal airway boundaries would provide more accurate findings and be beneficial for improving the airway for better respiratory function.




 PDF
PDF Citation
Citation Print
Print



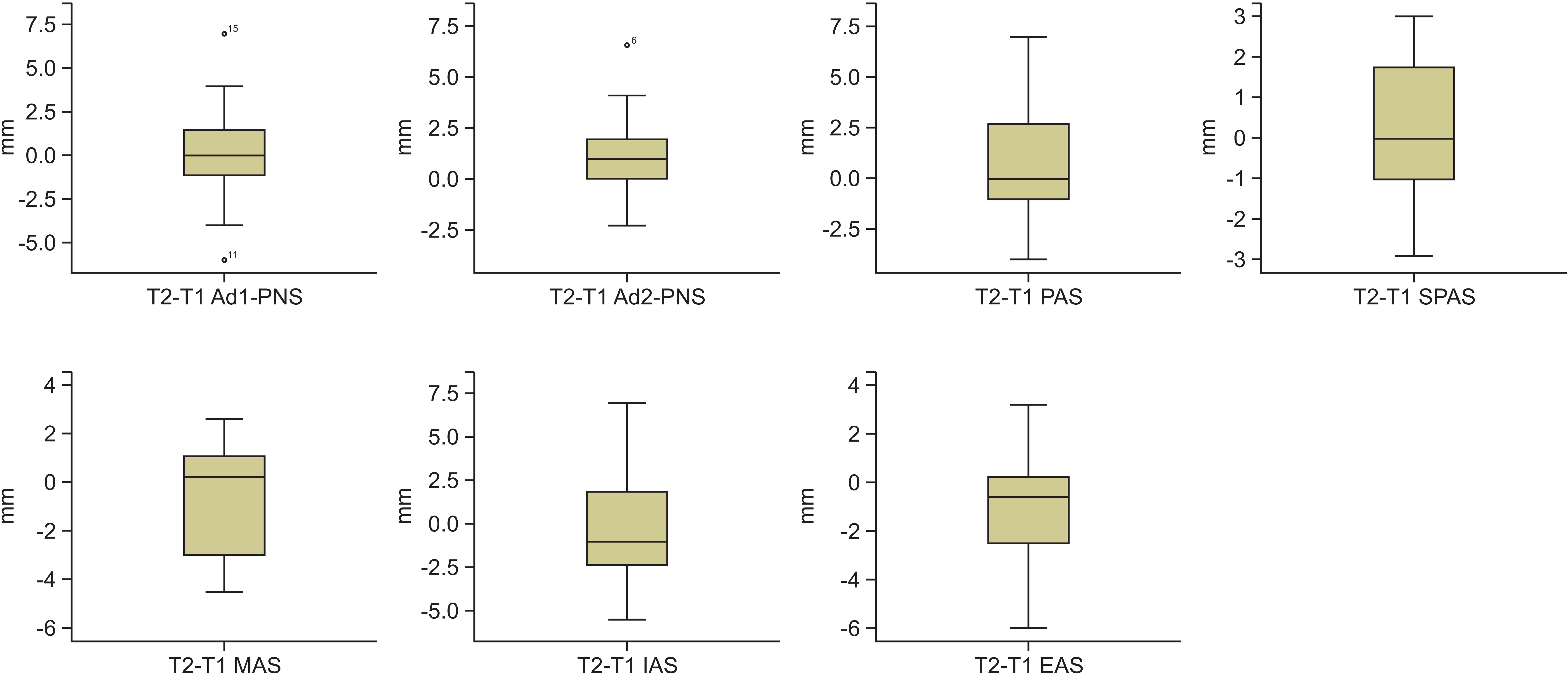
 XML Download
XML Download