1. Antonio-Zancajo L, Montero J, Albaladejo A, Oteo-Calatayud MD, Alvarado-Lorenzo A. 2020; Pain and oral-health-related quality of life in orthodontic patients during initial therapy with conventional, low-friction, and lingual brackets and aligners (Invisalign): a prospective clinical study. J Clin Med. 9:2088. DOI:
10.3390/jcm9072088. PMID:
32635196. PMCID:
PMC7408790. PMID:
ae922d85ec61459999b0394a5d12238d.

2. Erbe C, Hornikel S, Schmidtmann I, Wehrbein H. 2011; Quantity and distribution of plaque in orthodontic patients treated with molar bands. J Orofac Orthop. 72:13–20. DOI:
10.1007/s00056-010-0001-4. PMID:
21484542.

4. Manuelli M, Marcolina M, Nardi N, Bertossi D, De Santis D, Ricciardi G, et al. 2019; Oral mucosal complications in orthodontic treatment. Minerva Stomatol. 68:84–8. DOI:
10.23736/S0026-4970.18.04127-4. PMID:
30854838.

5. Bishara SE, Ostby AW. 2008; White spot lesions: formation, prevention, and treatment. Semin Orthod. 14:174–82. DOI:
10.1053/j.sodo.2008.03.002.

6. Walsh LJ, Healey DL. 2019; Prevention and caries risk management in teenage and orthodontic patients. Aust Dent J. 64 Suppl 1:S37–45. DOI:
10.1111/adj.12671. PMID:
31144319.

7. Lacerda Rangel Esper MÂ, Junqueira JC, Uchoa AF, Bresciani E, Navarro RS, Nara de Souza Rastelli A, et al. 2019; Photodynamic inactivation of planktonic cultures and Streptococcus mutans biofilms for prevention of white spot lesions during orthodontic treatment: an in vitro investigation. Am J Orthod Dentofacial Orthop. 155:243–53. Erratum in: Am J Orthod Dentofacial Orthop 2019;155:458. DOI:
10.1016/j.ajodo.2018.03.027. PMID:
30712696.
8. Beyth N, Houri-Haddad Y, Baraness-Hadar L, Yudovin-Farber I, Domb AJ, Weiss EI. 2008; Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials. 29:4157–63. DOI:
10.1016/j.biomaterials.2008.07.003. PMID:
18678404.

9. Lim BS, Lee SJ, Lee JW, Ahn SJ. 2008; Quantitative analysis of adhesion of cariogenic streptococci to orthodontic raw materials. Am J Orthod Dentofacial Orthop. 133:882–8. DOI:
10.1016/j.ajodo.2006.07.027. PMID:
18538253.

10. Shah AG, Shetty PC, Ramachandra CS, Bhat NS, Laxmikanth SM. 2011; In vitro assessment of photocatalytic titanium oxide surface modified stainless steel orthodontic brackets for antiadherent and antibacterial properties against Lactobacillus acidophilus. Angle Orthod. 81:1028–35. DOI:
10.2319/021111-101.1. PMID:
22007663. PMCID:
PMC8903869.
12. Doudi M, Naghsh N, Heiedarpour A. 2011; The effect of silver nanoparticles on gram-negative bacilli resistant to extended-spectrum β-lactamase enzymes. Med Lab J. 5:44–51.
14. Bürgers R, Eidt A, Frankenberger R, Rosentritt M, Schweikl H, Handel G, et al. 2009; The anti-adherence activity and bactericidal effect of microparticulate silver additives in composite resin materials. Arch Oral Biol. 54:595–601. DOI:
10.1016/j.archoralbio.2009.03.004. PMID:
19375069.

15. Spacciapoli P, Buxton D, Rothstein D, Friden P. 2001; Antimicrobial activity of silver nitrate against periodontal pathogens. J Periodontal Res. 36:108–13. DOI:
10.1034/j.1600-0765.2001.360207.x. PMID:
11327077.

16. Ohira T, Yamamoto O, Iida Y, Nakagawa ZE. 2008; Antibacterial activity of ZnO powder with crystallographic orientation. J Mater Sci Mater Med. 19:1407–12. DOI:
10.1007/s10856-007-3246-8. PMID:
17914627.

19. International Organization for Standardization (ISO). 2009. ISO 10993-5:2009. Biological evaluation of medical devices- part 5: tests for in vitro cytotoxicity. ISO Copyright Office;Geneva:
20. Tavassoli-Hojjati S, Haghgoo R, Mehran M, Niktash A. 2012; Evaluation of the effect of fluoride gel and varnish on the demineralization resistance of enamel: an in vitro. J Iran Dent Assoc. 24:28–34.
21. Farhadian N, Usefi Mashoof R, Khanizadeh S, Ghaderi E, Farhadian M, Miresmaeili A. 2016; Streptococcus mutans counts in patients wearing removable retainers with silver nanoparticles vs those wearing conventional retainers: a randomized clinical trial. Am J Orthod Dentofacial Orthop. 149:155–60. Erratum in: Am J Orthod Dentofacial Orthop 2017;151:11. DOI:
10.1016/j.ajodo.2015.07.031. PMID:
26827971.
22. Arash V, Keikhaee F, Rabiee SM, Rajabnia R, Khafri S, Tavanafar S. 2016; Evaluation of antibacterial effects of silver-coated stainless steel orthodontic brackets. J Dent (Tehran). 13:49–54. PMID:
27536328. PMCID:
PMC4983565. PMID:
93db76e397d94eb4b16f3f304c3d33e2.
23. Ghorbanzadeh R, Pourakbari B, Bahador A. 2015; Effects of baseplates of orthodontic appliances with in situ generated silver nanoparticles on cariogenic bacteria: a randomized, double-blind cross-over clinical trial. J Contemp Dent Pract. 16:291–8. DOI:
10.5005/jp-journals-10024-1678. PMID:
26067732.

24. Poosti M, Ramazanzadeh B, Zebarjad M, Javadzadeh P, Naderinasab M, Shakeri MT. 2013; Shear bond strength and antibacterial effects of orthodontic composite containing TiO2 nanoparticles. Eur J Orthod. 35:676–9. DOI:
10.1093/ejo/cjs073. PMID:
23264617.

25. Jedrychowski JR, Caputo AA, Kerper S. 1983; Antibacterial and mechanical properties of restorative materials combined with chlorhexidines. J Oral Rehabil. 10:373–81. DOI:
10.1111/j.1365-2842.1983.tb00133.x. PMID:
6355413.

26. Bulut H, Türkün M, Türkün LS, Işiksal E. 2007; Evaluation of the shear bond strength of 3 curing bracket bonding systems combined with an antibacterial adhesive. Am J Orthod Dentofacial Orthop. 132:77–83. DOI:
10.1016/j.ajodo.2005.06.040. PMID:
17628254.

27. Kachoei M, Divband B, Rahbar M, Esmaeilzadeh M, Ghanizadeh M, Alam M. 2021; A novel developed bioactive composite resin containing silver/zinc oxide (Ag/ZnO) nanoparticles as an antimicrobial material against
Streptococcus mutans,
Lactobacillus, and
Candida albicans. Evid Based Complement Alternat Med. 2021:4743411. DOI:
10.1155/2021/4743411. PMID:
34697547. PMCID:
PMC8541865.
28. Garmasheva I, Kovalenko N, Voychuk S, Ostapchuk A, Livins'ka O, Oleschenko L. 2016; Lactobacillus species mediated synthesis of silver nanoparticles and their antibacterial activity against opportunistic pathogens in vitro. Bioimpacts. 6:219–23. DOI:
10.15171/bi.2016.29. PMID:
28265538. PMCID:
PMC5326670.

29. Sharma VK, Yngard RA, Lin Y. 2009; Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 145:83–96. DOI:
10.1016/j.cis.2008.09.002. PMID:
18945421.

31. Hernández-Sierra JF, Ruiz F, Pena DC, Martínez-Gutiérrez F, Martínez AE, Guillén Ade J, et al. 2008; The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine. 4:237–40. DOI:
10.1016/j.nano.2008.04.005. PMID:
18565800.

32. Cieplik F, Aparicio C, Kreth J, Schmalz G. 2022; Development of standard protocols for biofilm-biomaterial interface testing. JADA Found Sci. 1:100008. DOI:
10.1016/j.jfscie.2022.100008.

33. Kasraei S, Sami L, Hendi S, Alikhani MY, Rezaei-Soufi L, Khamverdi Z. 2014; Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor Dent Endod. 39:109–14. DOI:
10.5395/rde.2014.39.2.109. PMID:
24790923. PMCID:
PMC3978100.

35. Ahrari F, Eslami N, Rajabi O, Ghazvini K, Barati S. 2015; The antimicrobial sensitivity of Streptococcus mutans and Streptococcus sangius to colloidal solutions of different nanoparticles applied as mouthwashes. Dent Res J (Isfahan). 12:44–9. DOI:
10.4103/1735-3327.150330. PMID:
25709674. PMCID:
PMC4336971.

36. Prabha RD, Kandasamy R, Sivaraman US, Nandkumar MA, Nair PD. 2016; Antibacterial nanosilver coated orthodontic bands with potential implications in dentistry. Indian J Med Res. 144:580–6. DOI:
10.4103/0971-5916.200895. PMID:
28256467. PMCID:
PMC5345305.
37. Halimi SU, Bakar NFA, Ismail SN, Hashib SA. 2014; Electrospray deposition of titanium dioxide (TiO2) nanoparticles. AIP Conf Proc. 1586:57. DOI:
10.1063/1.4866730.

38. Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A. 2013; Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol. 87:1181–200. DOI:
10.1007/s00204-013-1079-4. PMID:
23728526. PMCID:
PMC3677982.

39. Kim S, Choi JE, Choi J, Chung KH, Park K, Yi J, et al. 2009; Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol In Vitro. 23:1076–84. DOI:
10.1016/j.tiv.2009.06.001. PMID:
19508889.

40. Lesniak A, Salvati A, Santos-Martinez MJ, Radomski MW, Dawson KA, Åberg C. 2013; Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J Am Chem Soc. 135:1438–44. DOI:
10.1021/ja309812z. PMID:
23301582.

41. Shahi S, Özcan M, Maleki Dizaj S, Sharifi S, Al-Haj Husain N, Eftekhari A, et al. 2019; A review on potential toxicity of dental material and screening their biocompatibility. Toxicol Mech Methods. 29:368–77. DOI:
10.1080/15376516.2019.1566424. PMID:
30642212.





 PDF
PDF Citation
Citation Print
Print



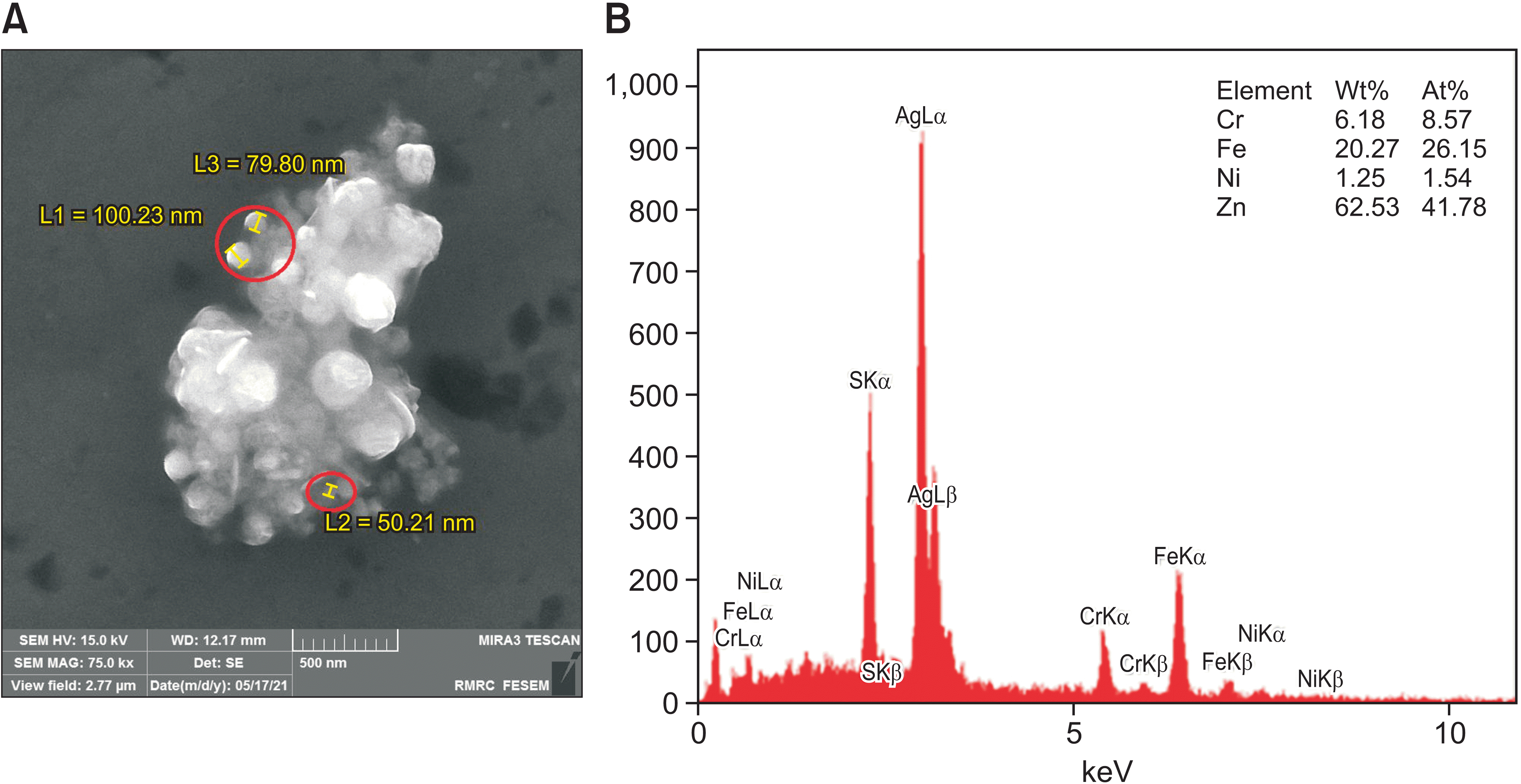
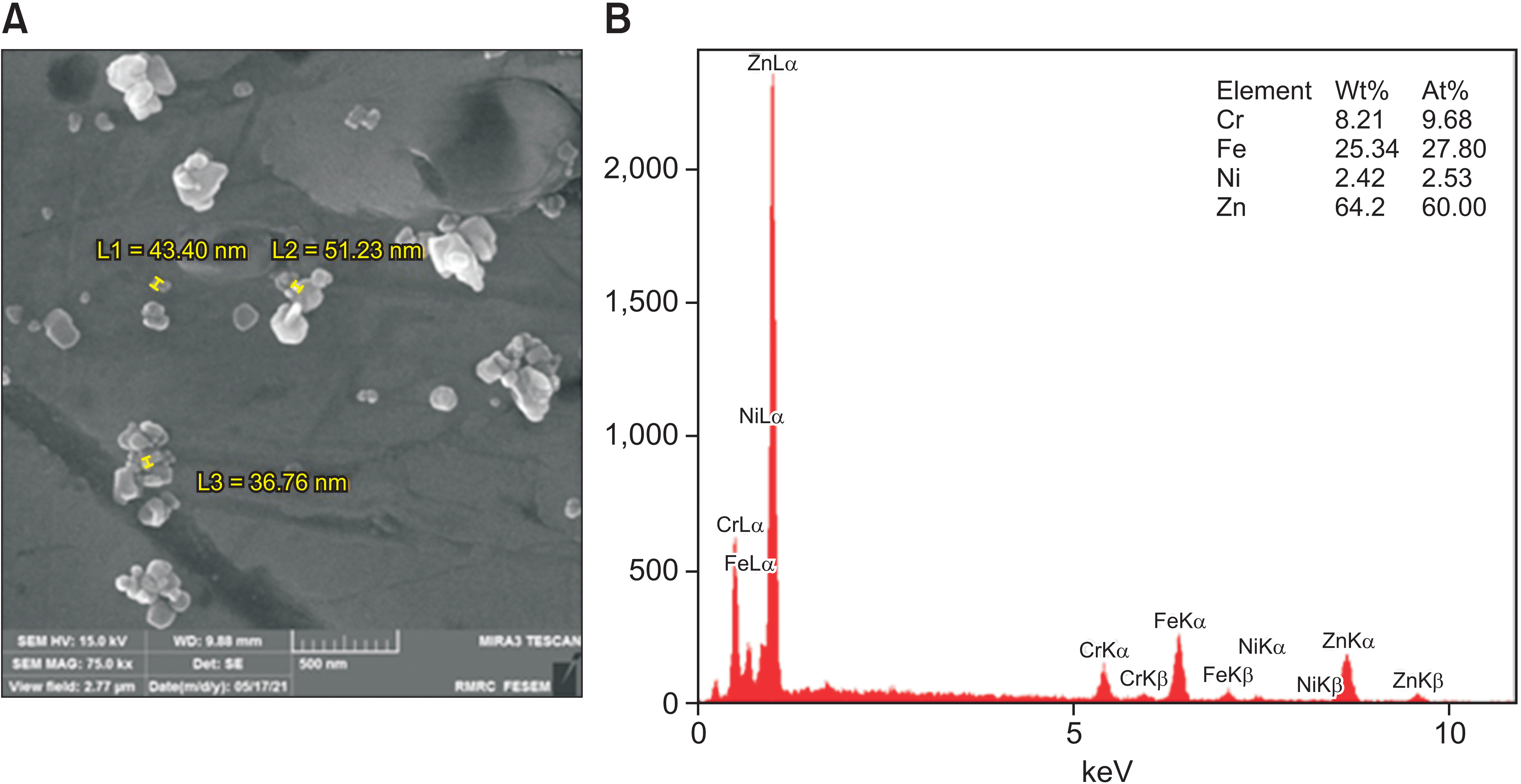
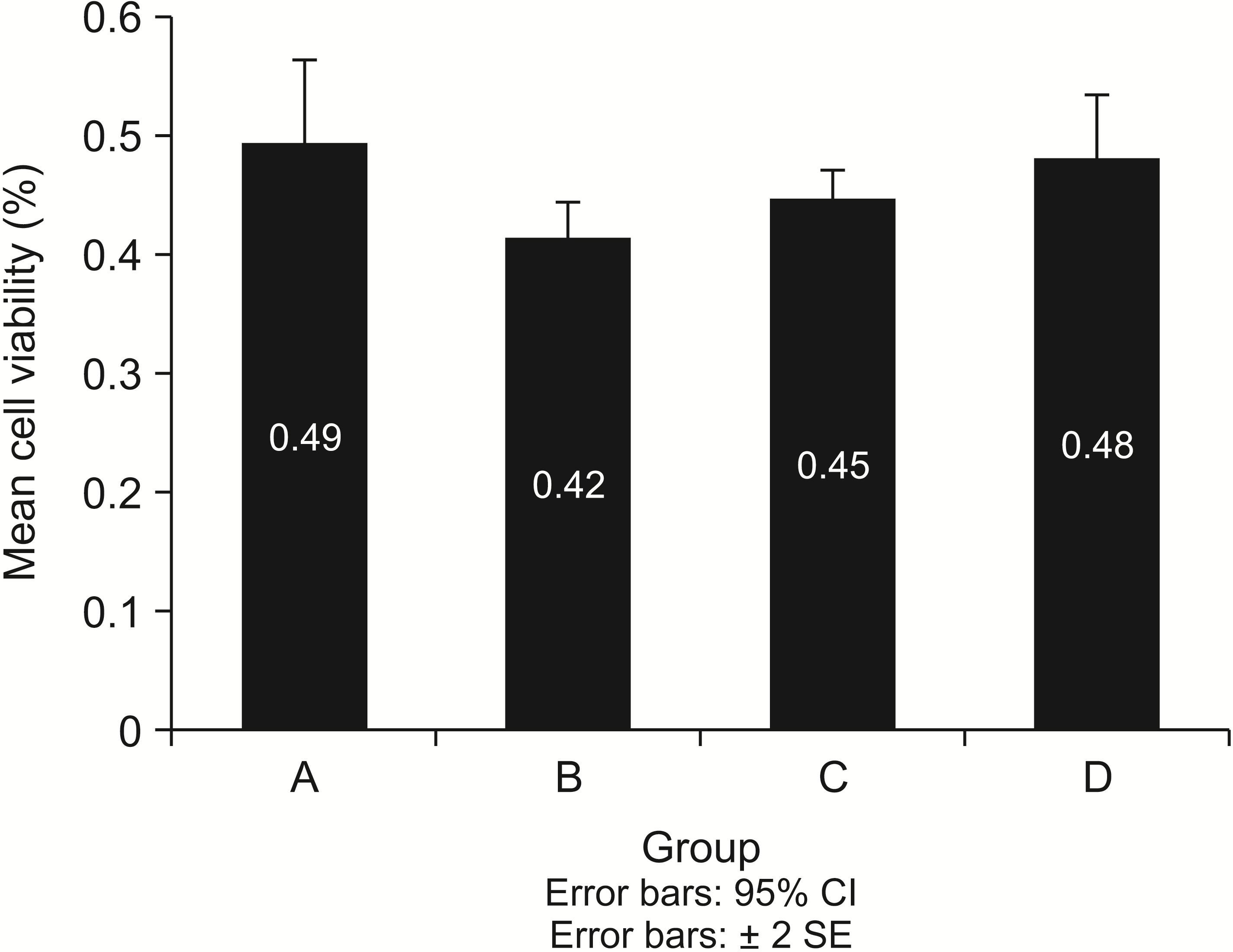
 XML Download
XML Download