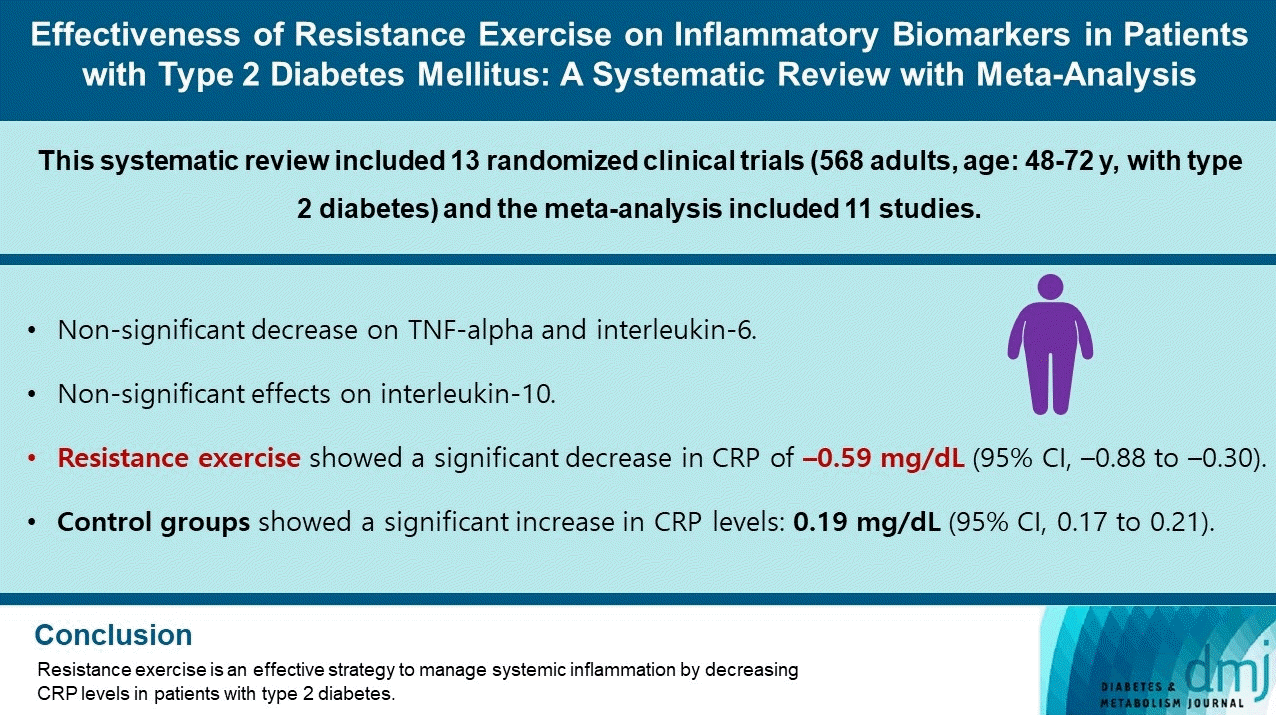
Abstract
Background
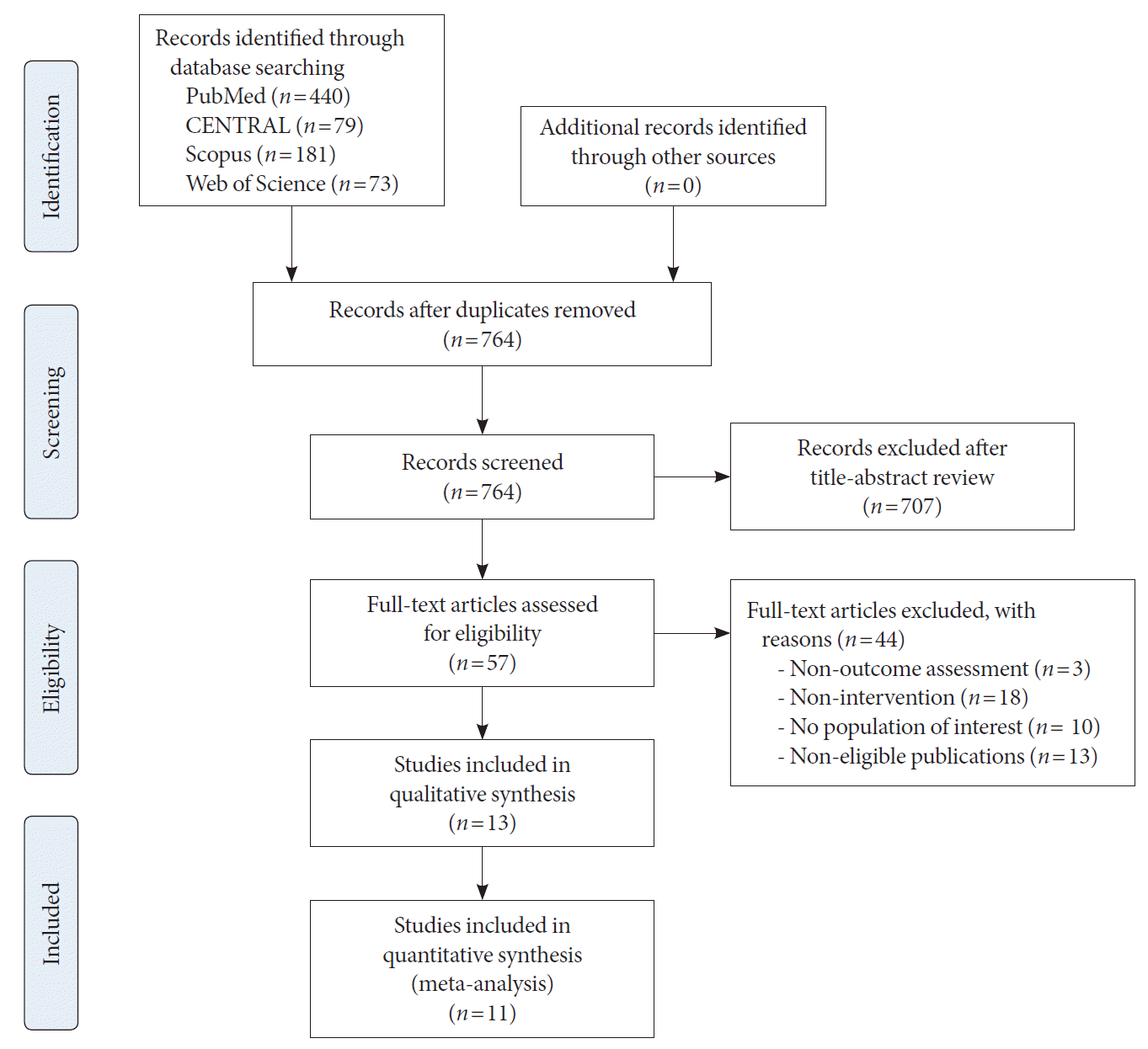
Methods
Results
SUPPLEMENTARY MATERIALS
Supplementary Table 2.
Supplementary Table 3.
Supplementary Table 4.
Supplementary Table 6.
Supplementary Table 7.
Supplementary Table 8.
Supplementary Fig. 1.
Supplementary Fig. 2.
Supplementary Fig. 3.
Supplementary Fig. 4.
Supplementary Fig. 5.
Notes
AUTHOR CONTRIBUTIONS
Conception or design: R.F.R., S.M.C., B.B.P.
Acquisition, analysis, or interpretation of data: R.F.R., S.M.C., M.G.M., A.E.M., V.M.V.
Drafting the work or revising: R.F.R., S.M.C.
Final approval of the manuscript: R.F.R., S.M.C., B.B.P., M. G.M., A.E.M., V.M.V.
FUNDING
This study was funded by the European Regional Development Fund. The funding source had no such involvement or restrictions regarding publication. Bruno Bizzozero-Peroni is supported by a grant from the Universidad de Castilla-La Mancha co-financed by the European Social Fund (2020-PREDUCLM-16746). Arthur Eumann Mesas was supported by a ‘Beatriz Galindo’ contract (BEAGAL18/00093) by the Spanish Ministry of Education, Culture and Sport.
REFERENCES
Fig. 2.
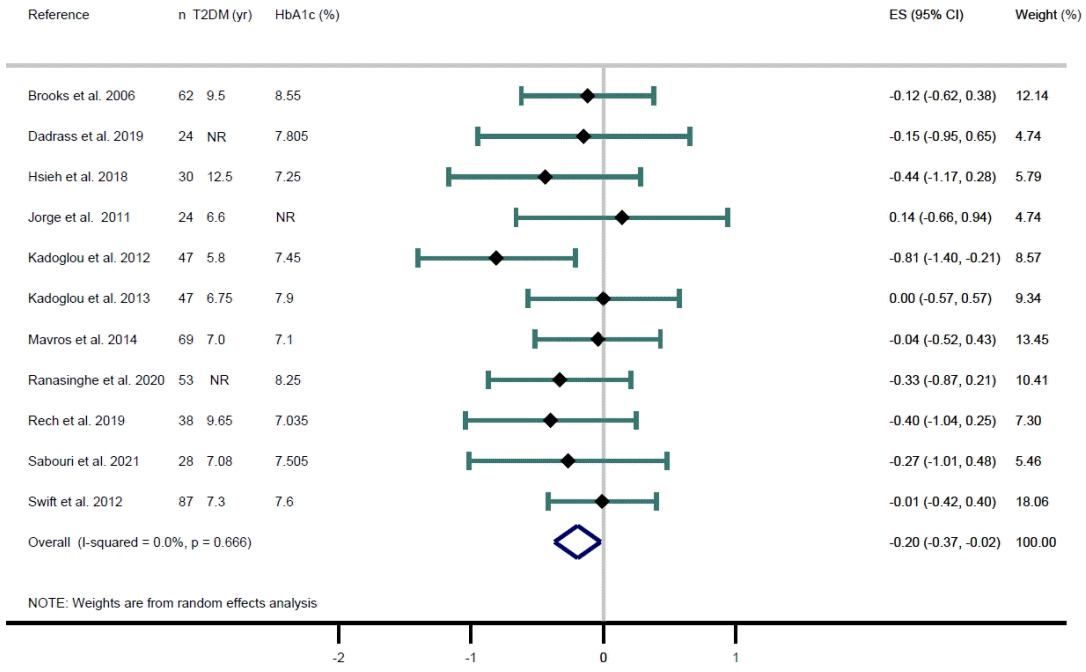
Fig. 3.
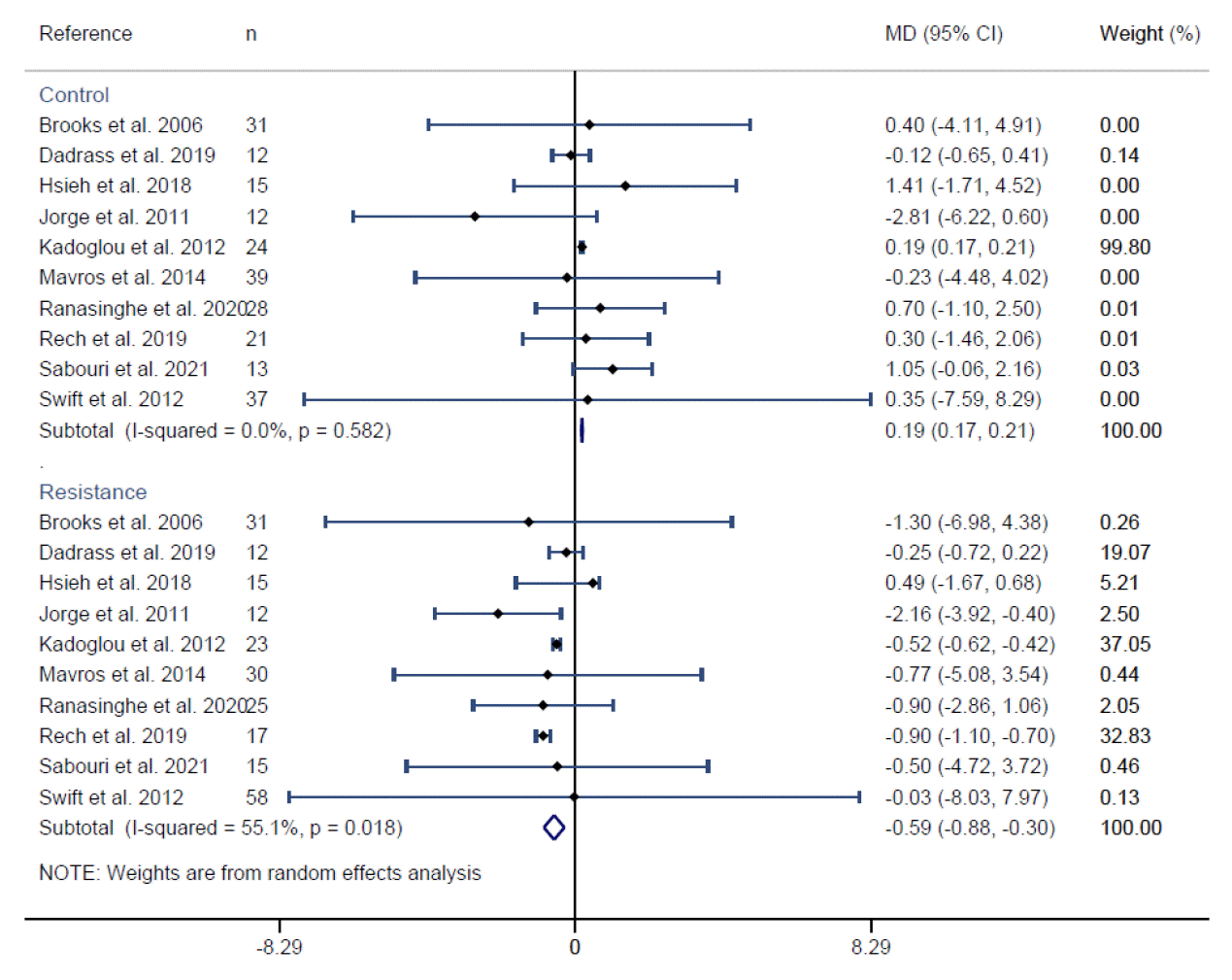
Fig. 4.
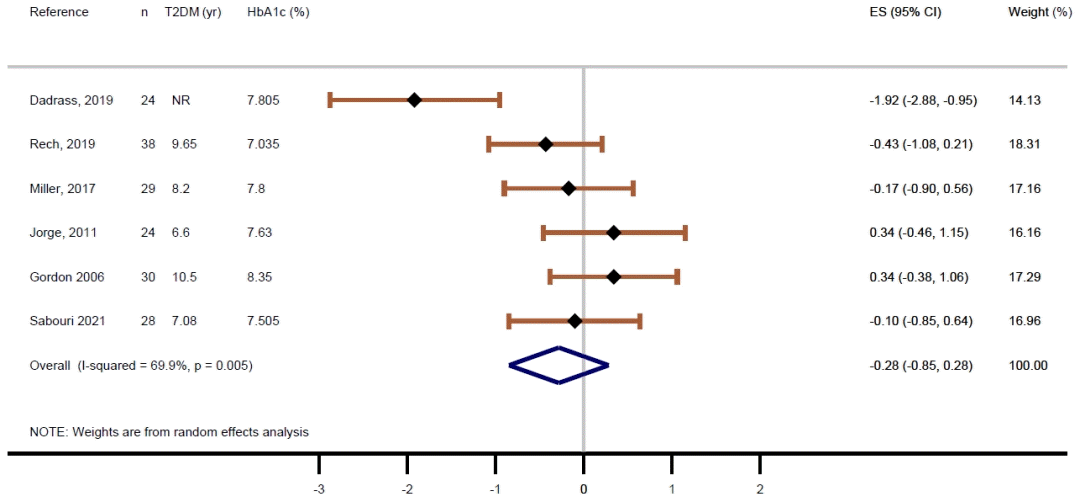
Fig. 5.
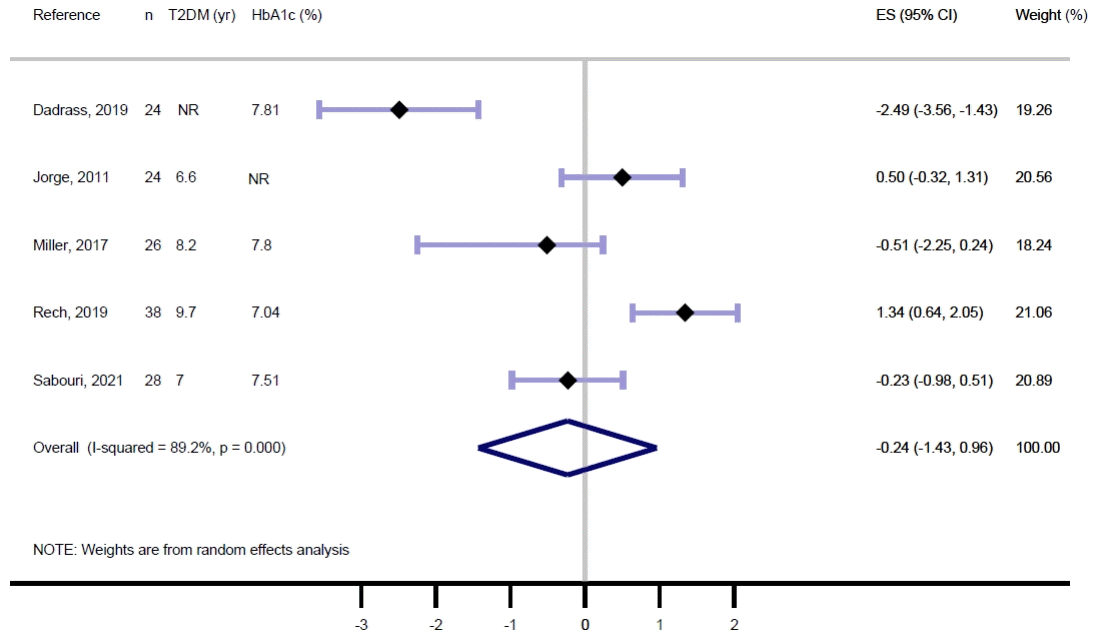
Table 1.
| Study | Sample size (% women) | Age, mean±SD, yr | Characteristics of the intervention | Comparison | Outcome | Main results | ||
|---|---|---|---|---|---|---|---|---|
| Gordon et al. (2006) [24] | n=30 (50%) | RE=67±2 | RE protocol | CG | TNF-α | No significant increase of TNF-α in the RE group, and no significant change in the CG. | ||
| RE=15 | CG=67±2 | →3×wk; 16 wk | → Telephone monitoring and face-to-face visits | |||||
| CG=15 | → Session: warm-up (5 min gentle walk+stretching); training (upper and lower body-machines)+T2DM related care | |||||||
| → Intensity: 60%–65% of 1 RM and progress 75%–80% of 1 RM at the end of the first 4 wk (wk 8 and 16 re-evaluation of 1 RM) | ||||||||
| → 3 sets of 8 reps, 3 sec rest between reps and 1–2 min between sets | ||||||||
| Brooks et al. (2006) [25] | n=62 (35.5%) | RE=66±2 | RE protocol | RE | CRP | CRP decreased in favor of RE but not significantly. | ||
| RE=31 | CG=66±1 | → 3×wk; 16 wk | → 16 wk | |||||
| CG=31 | → Supervised session: warm-up (5 min)–workout (35 min-upper and lower body-machines)–cool down (5 min)+standard care | → Standard care (health, glycemic control, self-monitoring, physical activity, medications and medical visits) | ||||||
| → Intensity: 60%–80% of 1 RM (1–8 wk) and 70%–80% of 1 RM (10–14 wk) | ||||||||
| → 3 sets of 8 reps/machine | ||||||||
| Jorge et al. (2011) [29] | n=24 (62.5%) | (53.9±9.9) | RE protocol | CG | TNF-α | hs-CRP decreased significantly in all groups. TNF-α and IL-6 increased in the ST group but not significantly. | ||
| RE=12 | RE=54±8.9 | → 3×wk; 12 wk | → 3×wk; 12 wk | CRP | ||||
| CG=12 | CG=53±9.8 | → Supervised session: training (60 min circuit 7 full body exercises: large muscle groups) | → Stretching | IL-6 | ||||
| AE=52±8.7 | AE | |||||||
| ST+AE=58±9.8 | → 60 min, 3×wk, 12 wk | |||||||
| → Supervised session: cycling | ||||||||
| → Intensity: heart rate according to lactate threshold | ||||||||
| RE+AE | ||||||||
| → 3×wk, 12 wk | ||||||||
| → Same intensity and volume | ||||||||
| Kadoglou et al. (2012) [30] | n=47 (25.53%) | RE=62±5.4 | RE protocol | CG | CRP | hs-CRP decreased in favor of RE group when compared to CG but not significantly. | ||
| RE=23 | CG=65±4.3 | → 45 min (basal) and 60 min (progression), 3×wk; 12 wk | → 150 min/wk: advice+daily physical activity (walking, cycling, swimming) varying from low to high intensity | |||||
| CG=24 | → Supervised session: calisthenics (jumping, skipping, gymnastics, ball games); training (upper and lower body: machines)–calisthenics (idem) | |||||||
| → Intensity: 60%–80% of 1 RM, 2–3 sets of 6–8 repetitions/exercise | ||||||||
| → 8 exercises with 1 min rest between exercises and 3 min between sets | ||||||||
| Swift et al. (2012) [31] | n=87 (61%) | (57.3±8.1) | RE protocol | CG | CRP | All intervention groups showed lower levels of CRP when compared to CG but not significantly. | ||
| RE=50 | RE=59±8 | → 3×wk, 36 wk | → 36 wk. No exercise prescription. Weekly stretching and relaxation classes | |||||
| CG=37 | CG=59±8.6 | → Supervised session: workout (whole body) | AE | |||||
| AE=56±7.9 | → 12 kcal/kg/wk | |||||||
| RE+AE=57±7.8 | → Intensity: 2 sets of 4 exercises (upper body), 3 sets of 3 exercises (lower body) and 2 sets of abs and back | → Supervised session: alternating walking on treadmill and cycling | ||||||
| → Intensity: 50%–80% maximal oxygen uptake | ||||||||
| → Each exercise 10–12 repetitions | RE+AE | |||||||
| → AE (supervised): | ||||||||
| → Weight progressively increased when the participant completed 12 repetitions of all exercises in 2 consecutive sessions | -10 kcal/kg/wk | |||||||
| - Intensity: 50%–80% maximal oxygen uptake | ||||||||
| → RE (supervised): | ||||||||
| -2*wk | ||||||||
| -9 exercises×1 set, incremental load | ||||||||
| Kadoglou et al. (2013) [32] | n=47 (27.78%) | RE=56±5.3 | RE protocol | CG | CRP | hs-CRP decreased in favor of AE, RE+AE when compared to RE and CG groups. | ||
| RE=23 | CG=58±7.2 | → Gradual increase of duration first 4 wk, then, 60 min 4×wk, 24 wk | → Physical activity (150 min/wk): low to moderate intensity walking | |||||
| CG=24 | AE=58±5.4 | → Supervised session: calisthenics (10 min)–training (8 upper and lower body exercises) | AE | |||||
| RE+AE= 58±6.5 | → Gradual duration over 4 wk; then 60 min, 4×wk, 6 mo | |||||||
| → Intensity: 60%–80% of 1 RM | → Supervised session: warm-up (10 min)–workout (45 min-walk- or run-on treadmill, bike or calisthenics)–cool down (5 min) | |||||||
| → 2–3 sets of 8–10 reps/exercise and 8 exercises | → Intensity: gradual over 4 wk, then 60%–75% of HRmax | |||||||
| RE+AE | ||||||||
| → 4×wk (1 of AE+1 of ST+2 of AE+ST), 6 mo | ||||||||
| → Session: warm up (10 min)–ST+AE training (45 min)–cool down (5 min) | ||||||||
| → Intensity: gradually increased during first 4 wk | ||||||||
| Mavros et al. (2014) [33] | n=69 (47.7%) | (68.2±5.7) | RE protocol | CG | CRP | CRP showed a not significant diminish in the RE group compared to CG. | ||
| RE=30 | RE=67±4.9 | → 3×wk, 48 wk | → 3×wk, 48 wk | |||||
| CG=39 | CG=69±6.3 | → Supervised session: training: concentric contraction (+fast as possible) and eccentric contraction 4 sec–whole body | → Non-progressive and low-intensity exercise, under supervision+regular care | |||||
| → Intensity: 80% of 1 RM (re-evaluation every 4 wk), 3 sets of 8 repetitions (2 sets of 8 for hip flexion, extension, and abduction) | ||||||||
| Hsieh et al. (2018) [34] | n=30 (63.3%) | RE=71±4.2 | RE protocol | CG | CRP | CRP levels decreased in RE group and increased in the CG but not significantly. | ||
| RE=15 | CG=72±4.5 | → 3×wk, 12 wk | → 3×wk, 12 wk | |||||
| CG=15 | → Supervised session: training–8 full body exercises (machines and body weight) | → Standard care and maintaining their daily activities and lifestyle | ||||||
| → 3 sets of 8–12 repetitions. Rest 60–90 sec between sets | ||||||||
| → Intensity: 40%–50% of 1 RM or 12–13 on the Borg scale | ||||||||
| → Progression 75% of 1 RM or 14–16 on the Borg scale at wk 12 | ||||||||
| Miller et al. (2017) [35] | 1) n=29 (44.8%) | RE+CD= 68±5.2 | RE+control diet (CD), (ST+CD) | CG+CD | TNF-α | No significant changes (TNF-α, IL-6, IL‑10) after 3, 6, 9, or 12 mo, except for TNF-α that showed a significant decrement at 9 and 12 mo for ST+CD when compared to CG+CD, and IL-10, which diminish significantly after 9 mo in the RE+CD group compared to CG+CD. | ||
| RE=16 | CG+CD= 67±5.3 | First phase (6 mo): 45 min, 3×wk, 24 wk | → 1st phase (6 mo): static pedalling without load+5 min of static stretching+healthy eating plan (evaluation every 2 wk) | IL-10 | ||||
| CG=13 | → Session: training (9 exercises–weights and machines)+healthy eating plan (evaluation every 2 wk) | IL-6 | ||||||
| 2) n=26 (44.8%) | → Intensity: 75%–85% of 1 RM, 3 sets of 8–10 repetitions | → 2nd phase (6 mo): static pedalling without load+5 min of static stretching | ||||||
| RE=14 | → Second phase (6 mo): 45 min, 3×wk, 24 wk | |||||||
| CG=12 | → Session: training (home exercises with dumbbells) | |||||||
| → Intensity: 60% of 1 RM | ||||||||
| → 3 sets of 8–10 repetitions | ||||||||
| Dadrass et al. (2019) [36] | n=24 (0%) | RE protocol | RE protocol | CG | IL-6 | IL-6 decreased significantly in all groups. TNF-α decreased significantly in ST+VitD and there were no changes in CG and GVitD. CRP decreased not significantly in all groups. | ||
| RE=12 | RE=55±5.9 | → 70 min, 3×wk, 12 wk | → Normal daily life+oral capsules (placebo) every 2 wk for 12 wk | TNF-α | ||||
| CG=12 | CG=53±8 | → Session: warm-up (10 min–walking and stretching); training (50 min–whole body-body weight and machines)–cool-down (10 min–stretching) | RE+VitD | CRP | ||||
| RE+VitD= 54±8 | → Intensity: 55% of 1 RM (1st mo); 65% of 1 RM (2nd mo) and 75% of 1 RM (3rd mo). Recalculated (4 and 8 wk) | → RE+VitD every 2 wk for 12 wk | ||||||
| VitD= 54±6.6 | → 10 exercises, 3 sets of 10 reps/exercise with 90 sec rest between sets and 30 sec between exercises | VitD | ||||||
| → Oral capsules (placebo) every 2 wk for 12 wk | → VitD every 2 wk for 12 wk | |||||||
| Rech et al. (2019) [26] | n=38 (47.4%) | RE=71±7.4 | → 3×wk, 12 ek | CG | TNF-α | TNF-α and ratio TNF-α/IL-10 decreased significantly in both groups. IL-6 and IL‑10 increased, and CRP decreased, but there were no significant interactions reported. | ||
| RE=17 | CG=68±6.5 | → Session: warm-up (on treadmill); training (functional exercises [i.e., step, squats]; and traditional (i.e., whole body using body weight and machines])–stretching exercises | → 45 min/session: joint mobilization+static stretching of large muscle groups (20–30 sec) | IL-10 | ||||
| CG=21 | → Intensity: f.e., intensity controlled through an scale (progress from 2 to 3 sets/exercise with 10–15 repetitions and 1 min rest); t.e., (progress from 2 to 3 sets/exercise and 12 to 10 repetitions, 1 min rest and 1.3 min progress) | CRP | ||||||
| IL-6 | ||||||||
| Ranasinghe et al. (2021) [27] | n=53 (53%) | (50.1±8.7) | RE protocol | CG | CRP | RE and AE decreased not significantly CRP levels when compared to CG. | ||
| RE=25 | RE=49±9 | → 60–75 min, 2×wk, 12 wk | → Usual clinic visits and contact once every 2 wk for 12 wk | |||||
| CG=28 | CG=49±7 | → Supervised session: warm-up (10 min–treadmill walking); workout (whole body–body weight, free weights, and machines)–cool down (10 min–dynamic and static stretching) | AE | |||||
| AE=52±9.8 | → 75 min, 2×wk, 12 wk | |||||||
| → Intensity: 50%–60% of 1 RM. Increase weight by 5% every 2 wk | → Supervised session: warm up (10 min–treadmill walking)–workout (circuit-walking, step, exercise bike)–cool down (10 min–static stretching) | |||||||
| → 3 sets of 8–10 repetitions | → Intensity: 60%–75% HRmax | |||||||
| Sabouri et al. (2021) [28] | n=28 (45.8%) | (50.1±8.7) | RE protocol | CG | TNF-α | TNF-α, IL-6, and CRP showed a significant decrease in RE groups. CG showed a nonsignificant decrease in TNF-α and IL‑6 and nonsignificant increase of CRP. | ||
| RE=15 | RE=51±4.5 | → 3×wk, 12 wk | → No intervention, 12 wk | CRP | ||||
| CG=13 | CG=52±3.2 | → Supervised session: 3 sets of 8 reps max RM (upper and lower body)+3 sets of 15 reps (abdomen), 1 min rest between sets | HIIT | IL-6 | ||||
| → 3×wk, 12 wk | ||||||||
| → Supervised session: at cycloergometer with protocol: 10×60 sec at 85%–90% of HRmax, 1 min of active recovery | ||||||||
| → Intensity: maximum weight for 8 repetitions | HIIT+ST | |||||||
| → 3×wk, 12 wk | ||||||||
| → Supervised session: first ST, then HIIT | ||||||||
SD, standard deviation; RE, resistance exercise; CG, control group; T2DM, type 2 diabetes mellitus; RM, repetition maximum; TNF-α, tumor necrosis factor alpha; CRP, C-reactive protein; AE, aerobic exercise; IL-6, interleukin-6; hs-CRP, high-sensitivity C-reactive protein; HRmax, heart rate max; CD, control diet; IL-10, interleukin-10; VitD, vitamin D; HIIT, high intensity interval training.
Appendix
Appendix 1.
PRISMA checklist
| Section and Topic | Item # | Checklist item | Location where item is reported | |
|---|---|---|---|---|
| TITLE | ||||
| Title | 1 | Identify the report as a systematic review. | 1, title | |
| ABSTRACT | ||||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | 2 | |
| INTRODUCTION | ||||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | 3 | |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | 3-4 | |
| METHODS | ||||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | 4-5 | |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | 4, Supplementary Table 1 | |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | Supplementary Table 1 | |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | 4-5 | |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | 5-6 | |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | 4, 6 | |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | 4, 6 | ||
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | 5 | |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | 6 | |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | 6 | |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | 6 | ||
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | 6 | ||
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | 6 | ||
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g. subgroup analysis, meta-regression). | 6 | ||
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | 6 | ||
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | NA | |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | 5-6 | |
| RESULTS | ||||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | 7, Fig. 1 | |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | Supplementary Table 2 | ||
| Study characteristics | 17 | Cite each included study and present its characteristics. | 7, 8, Table 1 | |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | 8, Supplementary material | |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | 8, 9, Figs. 2, 3 | |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | 8, 9, Supplementary material | |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | Figs. 2, 3 | ||
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | 9, Supplementary material | ||
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | Supplementary material | ||
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | NA | |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | Supplementary Table 3 | |
| DISCUSSION | ||||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | 9 | |
| 23b | Discuss any limitations of the evidence included in the review. | 10-11 | ||
| 23c | Discuss any limitations of the review processes used. | 11 | ||
| 23d | Discuss implications of the results for practice, policy, and future research. | 9-11 | ||
| OTHER INFORMATION | ||||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | Title, 2 | |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | 2 (PROSPERO register) | ||
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | NA | ||
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | Title | |
| Competing interests | 26 | Declare any competing interests of review authors. | Title | |
| A vailability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | NR | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download