Abstract
Background and Purpose
In patients with acute ischemic stroke (AIS) using a direct oral factor-Xa anticoagulant (DOAC) during the last 48 hours, a fixed plasma heparin-calibrated anti-Xa activity (0.5 IU/mL) was proposed as a threshold below which patients could be eligible for thrombolysis and/or thrombectomy. Besides, specific DOAC-calibrated anti-Xa thresholds up to 50 ng/mL have been proposed. However, specific DOAC assays are not widely available contrarily to low-molecularweight heparin (LMWH) anti-Xa activity. We developed and validated a nomogram for predicting apixaban and rivaroxaban concentrations based on LMWH anti-Xa assay.
Methods
Our prospective study included apixaban (n=325) and rivaroxaban (n=276) patients. On the same sample, we systematically measured specific DOAC concentration and LMWH anti-Xa activity, using STA®-Liquid-Anti-Xa (Stago) and specific DOAC- or LMWH-calibrators, respectively. The nomogram was built using quantifiable values for both assays on the derivation cohorts with a log-linear regression model. Model performances including sensitivity, specificity, and true positive rate for different thresholds were checked on the validation cohorts.
Results
The models built from the derivation cohorts predicted that values <30 ng/mL and <50 ng/ mL DOAC thresholds corresponded to LMWH-anti-Xa values <0.10 IU/mL and <0.64 IU/mL for apixaban; <0.10 IU/mL and <0.71 IU/mL for rivaroxaban. The model accurately predicted apixaban/ rivaroxaban concentrations in the validation cohort.
Conclusions
This easy-to-use nomogram, developed with our reagent, allowed accurately predicting DOAC concentrations based on LMWH-anti-Xa results in emergency situations such as AIS when drug-specific assessments are not rapidly available. Using DOAC <50 ng/mL equivalent threshold, instead of the fixed LMWH <0.5 IU/mL one, would allow proposing thrombolysis to more patients.
The number of patients with atrial fibrillation receiving direct oral factor-Xa anticoagulants (DOACs) to prevent stroke is rising steadily. Among patients presenting with acute ischemic stroke (AIS), prompt recanalization is a major challenge to improve functional outcomes [1]. In AIS patients on DOAC, mechanical thrombectomy (MT) is preferably encouraged when applicable. However, some DOAC patients may be eligible to intravenous thrombolysis (IVT) alone or in combination with MT. According to recent European Stroke Organization (ESO) guidelines, for patients with AIS of <4.5 hours duration, who used a factor Xa inhibitor during the last 48 hours before stroke onset and who have a heparin calibrated anti-Xa activity <0.5 IU/mL, IVT could be performed [2]. Other authors suggested that plasma DOAC-calibrated anti-Xa results (expressed in ng/mL) might be useful for decision-making [3-5]: the maximal DOAC threshold of 50 ng/mL was proposed. DOAC-calibrated anti-Xa activity assays are the tests of choice for the quantification of direct factor Xa inhibitors (e.g., rivaroxaban and apixaban) in daily practice [6]. One major drawback is that such assays are not available everywhere all the time with rapid turnaround time, contrarily to low-molecular-weight heparin (LMWH)/heparin calibrated anti-Xa assays (IU/mL). Both chromogenic assays are based on the same principle: inhibition of exogenous factor Xa, using the same reagents; they differ by specific dilution conditions, calibrations, and chromogen detection kinetics [6]. Consequently, the above mentioned fixed anti-Xa activity threshold (0.5 IU/mL) corresponds to variable DOAC concentrations depending on the DOAC drug and the assay [7]. Thus, it could prevent eligible patients with DOAC concentrations <50 ng/mL from IVT. It could also explain why the percentage of patients on DOAC receiving IV thrombolysis is rather low [1]. Previous studies proposed tools to convert heparin anti-Xa activity to DOAC concentrations [8-14]; however, most of them are complex to implement in clinical practice. Therefore, we sought to develop and validate a practical nomogram for the prediction of apixaban and rivaroxaban concentrations based on LMWH anti-Xa assay, allowing labs to quickly quantify anti-Xa DOACs when specific assays are lacking.
Our prospective study was conducted between May 2018 and January 2022 and included all consecutive patients on apixaban or rivaroxaban who were referred to our accredited hospital laboratory for hemostasis testing prior to thrombolysis or for thrombophilia screening (Lariboisière University Hospital, Assistance Publique—Hôpitaux de Paris [AP-HP]). We collected demographic data and anticoagulant treatment. We systematically measured specific DOAC concentration and LMWH anti-Xa activity on the same plasma aliquot. Both DOAC concentrations (expressed in ng/mL) and LMWH-anti-Xa level (IU/mL) were measured using STA®-Liquid-Anti-Xa (Stago, Asnières, France) on two STA R MAX (Stago) analyzers with specific set-up tests according to the manufacturer’s recommendations. Calibrations were performed using plasma calibrators (apixaban and rivaroxaban calibrators [Stago] for DOACs and Multi-HEP® [Stago] for LMWH). Dedicated quality-controls were used. The measuring ranges were 20–500 ng/mL for DOACs, 0.1–2.0 IU/mL for LMWH. Five reagent and calibrator batches were used over the study period. Each nomogram was built using a derivation and a validation cohort of patients on apixaban or rivaroxaban. Samples were randomly assigned to the derivation or the validation cohort, in a 50:50 ratio. The nomogram was built using quantifiable values for both assays on the derivation cohort, with a loglinear regression model, then used to determine LMWH thresholds ensuring, with a 95% confidence interval (CI), DOAC concentration below clinically relevant thresholds, i.e., 30, 50, 75, and 100 ng/mL. Model performances (sensitivity, specificity, and true positive rate [TPR]) were checked on the validation cohort, using the percentage of values correctly predicted (belonging to the 95% prediction interval, or for thresholds, correctly classified). All analyses were done using the R software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria). This study was approved by the “Bureau de la Protection des données” of the Hospital Group Saint-Louis-Lariboisière (AP-HP, HUSLSLRBFW). Patients were systematically informed that their anonymized laboratory data could be used for observational studies according to AP-HP ethical policy unless they objected to it. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
During the study period, we measured both specific DOAC anti-Xa levels and LMWH in samples from 601 patients: 325 patients (mean age 63±16 years, males 60%) on 5- or 2.5-mg apixaban b.i.d. and 276 patients (mean age 61±17 years, males 63%) on 20- or 15-mg rivaroxaban o.d. The derivation cohort comprised 160 and 138 samples from patients on apixaban or rivaroxaban, respectively. Apixaban (n=34) or rivaroxaban (n=82) samples with LMWH anti-Xa values out of the measuring range were discarded to build the nomogram to convert LMWH anti-Xa level into specific anti-Xa DOAC concentration (Table 1). We observed an exponential relationship between LMWH anti-Xa values and DOAC concentrations (Figure 1). The validation cohort included 165 and 138 samples from patients on apixaban or rivaroxaban, respectively. LMWH values up to 2.0 IU/mL accurately predicted DOAC levels for apixaban and for rivaroxaban (Figure 1). Moreover, we analyzed seven additional patients as controls who had received apixaban and were changed to LMWH (tinzaparin or enoxaparin at therapeutic dose) within three days before sampling when they were admitted to hospital. In these seven patients, anti-Xa values measured at LMWH peak level were the sum of anti-Xa activities of both LMWH and residual apixaban in plasma sample: in this special case, predicted apixaban anti-Xa value was logically higher than the true one, out of the prediction interval (Figure 1).
Finally, we evaluated in the validation cohort the model performances for different DOAC thresholds, namely 30, 50, 75, and 100 ng/mL in terms of sensitivity, specificity, and TPR, using the percentage of values correctly predicted (Table 2). For example, prediction of apixaban concentrations <30 ng/mL and <50 ng/mL corresponded to LMWH anti-Xa values <0.1 IU/mL (TPR 100% [15.8, 100]) and <0.64 IU/mL (TPR 94.7% [74.0, 99.9]), respectively; prediction of rivaroxaban concentrations <30 ng/mL and <50 ng/mL corresponded to LMWH anti-Xa values <0.1 IU/mL (TPR: 100% [15.8, 100]) and <0.71 IU/mL (TPR: 100% [89.4, 100]), respectively.
We built and validated a reliable nomogram accurately predicting apixaban or rivaroxaban concentration from LMWH anti-Xa activity up to 2.0 IU/mL performed according to the manufacturer’s recommendations, without additional sample dilution. This easyto-use tool showed robust performances for safely ruling out patients with DOAC concentration above different thresholds used in critical situations, especially 30 ng/mL or 50 ng/mL. Our study has several strengths. Firstly, our nomogram is an easy-to-use tool for clinicians, pathologists, or technicians, immediately providing the result without any calculation, thus limiting errors. The duration of anti-Xa test is 6 minutes, thus suitable for emergency. It does not require equation or conversion factors in contrast to previous studies proposing tools converting “uncalibrated” anti-Xa assay results into DOAC concentrations [7-12]. Secondly, we focused on the lower part of the curve to improve precision up to 100 ng/mL, contrary to most studies [7-12]. Noteworthy, we observed a clear exponential relationship between LMWH and DOAC anti-Xa activities in this measuring range and evidenced a much better fit than using a linear relationship reported in previous studies. These discrepancies between studies can be explained by differences among assays, deviations from manufacturer protocol (sample dilution, calibration curve mathematical processing), measuring range and/ or limited sample size. Given the characteristics of each antiXa assay, the development of a universal reliable nomogram (i.e., whatever the instrument/reagent combination) may lead to weak performances. Using a defined widely used anti-Xa assay, we provided good to excellent performances of the model from the validation cohort including a substantial number of patients for 30, 50, 75, and 100 ng/mL thresholds. However, this nomogram should be rebuilt for other assays.
We deliberately and prospectively limited the nomogram to anti-Xa value below 2.0 IU/mL, because it does not need additional plasma sample dilution, which is time-consuming even though automated. In case of anti-Xa >2.0 IU/mL, sample dilution in plasma may overcome this limitation to extend the DOAC result range if needed; however, the exponential relationship between LMWH anti-Xa activity (IU/mL) and DOAC concentration requires further investigation.
Our nomogram could be particularly useful in the numerous medical centers where specific anti-Xa DOAC assessments are not rapidly available although mandatory in emergency situations, such as life-threatening bleedings including the need for reversal, urgent surgery or acute stroke. Especially, the eligibility of DOAC patients to IVT may depend on DOAC concentrations, up to 50 ng/mL in case of last DOAC intake within 48 hours. Endovascular treatment can only be performed in a limited number of patients with intracranial proximal artery occlusion and number of centers. We showed here that the fixed 0.5 IU/mL anti-Xa threshold proposed in ESO guidelines corresponded to both apixaban and rivaroxaban levels of around 30 ng/mL [2]. Noteworthy, the current ESO guideline criteria of 0.5 IU/mL were propositions, without clinical evidence from any study. Thus, the determination of apixaban/rivaroxaban plasma concentrations using the nomogram could enable IVT in a larger proportion of patients taking DOAC, otherwise ineligible to this treatment [3,4]. A recent systematic review showed no increased risk of symptomatic intracerebral hemorrhage in selected AIS patients on DOAC [15] but data remain scarce. Thus, there is a need for reliable tools to safely expand the selection of eligible DOAC patients to IVT. Moreover, trials evaluating the benefit-to-risk ratio of IVT when the last DOAC intake is within 48 hours are required. Furthermore, we showed here that patients with LMWH-anti-Xa values <1.37 IU/mL and <1.55 IU/mL predicted apixaban and rivaroxaban concentration up to approximately 100 ng/mL, respectively, with excellent positive predictive values (TPR close to 100%). In patients presenting with life-threatening or uncontrolled bleeding, e.g., intracranial hemorrhage, the knowledge of DOAC levels >75 ng/mL might be helpful for reversal with andexanet-alpha decision-making.
Our study has several limitations. Firstly, our nomogram cannot be directly applicable to other anti-Xa assays; however, similar models could be easily built using other reagents. Secondly, knowing the anticoagulant treatment including bridge to LMWH is warranted to ensure correct prediction as clearly demonstrated in our study: indeed, anti-Xa activity measurement is not specific to rivaroxaban, apixaban, or LMWH as already previously stated [16]. Thirdly, our study was performed in only one center. Nevertheless, we used two analyzers and different reagent batches thus ensuring the robustness of the nomogram. Finally, DOAC concentrations were not measured using the reference method, i.e., high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS-MS): however, it has been well established that HPLC-MS-MS results are highly correlated with those obtained with specific DOAC anti-Xa assays [6] and HPLC-MS-MS is not suitable to routine analysis. Of note, we could not test edoxaban users since this DOAC is not licensed in France.
ACKNOWLEDGMENTS
AS and VS contributed to the design of the study; MD, PR MM recruited patients; CB, AS and VS acquired data, CB, VS and EC analysed and interpreted data; CB, VS, AS and EC wrote the draft. All authors revised and approved the manuscript.
References
1. Meinel TR, Branca M, De Marchis GM, Nedeltchev K, Kahles T, Bonati L, et al. Investigators of the Swiss stroke registry. Prior anticoagulation in patients with ischemic stroke and atrial fibrillation. Ann Neurol. 2021; 89:42–53.
2. Berge E, Whiteley W, Audebert H, De Marchis GM, Fonseca AC, Padiglioni C, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021; 6:I–LXII.
3. Touzé E, Gruel Y, Gouin-Thibault I, De Maistre E, Susen S, Sie P, et al. Intravenous thrombolysis for acute ischaemic stroke in patients on direct oral anticoagulants. Eur J Neurol. 2018; 25:747–e52.
4. Seiffge DJ, Traenka C, Polymeris AA, Thilemann S, Wagner B, Hert L, et al. Intravenous thrombolysis in patients with stroke taking rivaroxaban using drug specific plasma levels: experience with a standard operation procedure in clinical practice. J Stroke. 2017; 19:347–355.
5. Seiffge DJ, Poli S, Meinel TR, Wu T, Wilson D, Purrucker JC. Intravenous thrombolysis in patients taking direct oral anticoagulants (ESO IVT guidelines comment). Eur Stroke J. 2021; 6:445–446.
6. Douxfils J, Adcock DM, Bates SM, Favaloro EJ, Gouin-Thibault I, Guillermo C, et al. 2021 Update of the International Council for Standardization in Haematology Recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2021; 121:1008–1020.
7. Ebner M, Birschmann I, Peter A, Härtig F, Spencer C, Kuhn J, et al. Limitations of specific coagulation tests for direct oral anticoagulants: a critical analysis. J Am Heart Assoc. 2018; 7:e009807.
8. Beyer J, Trujillo T, Fisher S, Ko A, Lind SE, Kiser TH. Evaluation of a heparin-calibrated antifactor Xa assay for measuring the anticoagulant effect of oral direct Xa inhibitors. Clin Appl Thromb Hemost. 2016; 22:423–428.
9. Maier CL, Asbury WH, Duncan A, Robbins A, Ingle A, Webb A, et al. Using an old test for new tricks: measuring direct oral anti-Xa drug levels by conventional heparin-calibrated antiXa assay. Am J Hematol. 2019; 94:E132–E134.
10. Boissier E, Senage T, Babuty A, Gouin-Thibault I, Rozec B, Roussel JC, et al. Heparin anti-Xa activity, a readily available unique test to quantify apixaban, rivaroxaban, fondaparinux, and danaparoid levels. Anesth Analg. 2021; 132:707–716.
11. Willekens G, Studt JD, Mendez A, Alberio L, Fontana P, Wuillemin WA, et al. A universal anti-Xa assay for rivaroxaban, apixaban, and edoxaban measurements: method validation, diagnostic accuracy and external validation. Br J Haematol. 2021; 193:1203–1212.
12. Meihandoest T, Studt JD, Mendez A, Alberio L, Fontana P, Wuillemin WA, et al. Accuracy of a single, heparin-calibrated anti-Xa assay for the measurement of rivaroxaban, apixaban, and edoxaban drug concentrations: a prospective cross-sectional study. Front Cardiovasc Med. 2022; 9:817826.
13. Mithoowani S, Moffat KA, Gupta A, Carlino SA, Crowther MA. Low molecular weight heparin anti-Xa assays can identify patients with clinically important apixaban and rivaroxaban drug levels. Thromb Res. 2022; 215:1–4.
14. von Horn H, Rasmusson A, Söderblom L, Malmström RE, Antovic J. Using a low-molecular weight heparin-calibrated anti-factor Xa assay to assess the concentration of apixaban and rivaroxaban. Int J Lab Hematol. 2022; 44:163–167.
15. Shahjouei S, Tsivgoulis G, Goyal N, Sadighi A, Mowla A, Wang M, et al. Safety of intravenous thrombolysis among patients taking direct oral anticoagulants: a systematic review and meta-analysis. Stroke. 2020; 51:533–541.
16. Lessire S, Douxfils J, Pochet L, Dincq AS, Larock AS, Gourdin M, et al. Estimation of rivaroxaban plasma concentrations in the perioperative setting in patients with or without heparin bridging. Clin Appl Thromb Hemost. 2018; 24:129–138.
Figure 1.
Relationships between plasma LMWH anti-Xa activity (IU/mL) and apixaban (left) or rivaroxaban (right) concentration in the derivation cohort (at the top) and in the validation cohort (in the middle) and relationships between the effective measured concentration (using specific calibrators) and the DOAC concentration predicted by the model (at the bottom). Black dots, values of the derivation cohort; yellow dots, values below or above limits of quantification limits; green dots, values within the predicted interval in the validation cohort and those out (red dots); purple triangles, patients switched from apixaban to LMWH within the last three days, showing additive anti-Xa effects of both LMWH and residual apixaban in sample; blue solid line curve, the exponential relationship (dashed line 95% prediction interval); vertical yellow dashed lines, the lower and upper limits of anti-Xa quantification; horizontal yellow dashed lines, the lower limit of DOAC quantification. LMWH, low-molecular-weight heparin; DOAC, direct oral factor-Xa anticoagulant.
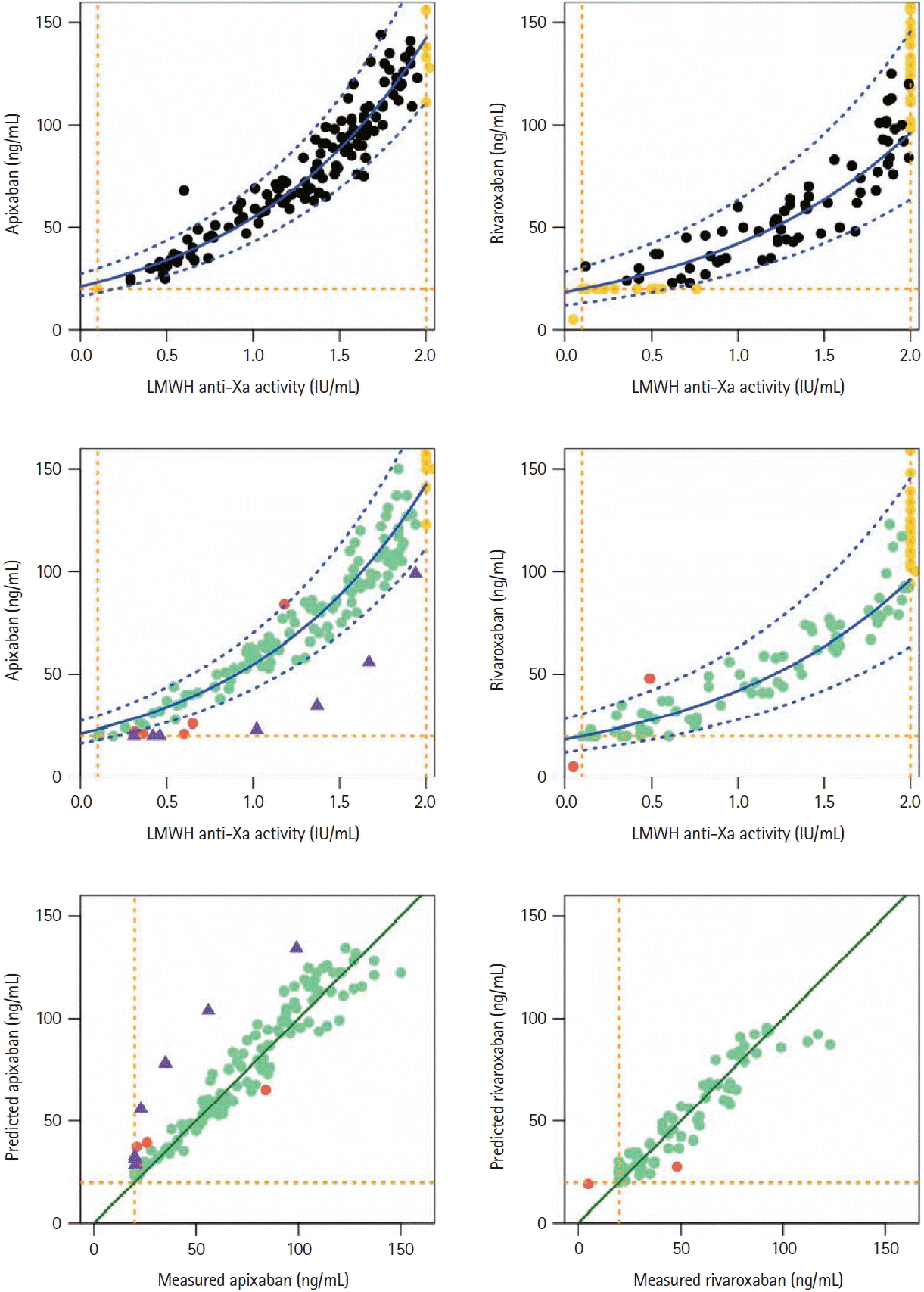
Table 1.
Nomogram converting LMWH anti-Xa activity (STA®-Liquid-Anti-Xa, Stago) into apixaban or rivaroxaban concentrations
| LMWH anti-Xa activity (IU/mL) |
Concentration (ng/mL) [95% CI] |
|
|---|---|---|
| Apixaban | Rivaroxaban | |
| ≤0.10 | ≤23 [18, 30] | ≤20 [13, 31] |
| 0.15 | 24 [19, 31] | 21 [14, 32] |
| 0.20 | 26 [20, 33] | 22 [14, 33] |
| 0.25 | 27 [21, 34] | 23 [15, 35] |
| 0.30 | 28 [22, 36] | 24 [15, 36] |
| 0.35 | 30 [23, 38] | 25 [16, 37] |
| 0.40 | 31 [24, 40] | 26 [17, 39] |
| 0.45 | 32 [25, 42] | 27 [18, 41] |
| 0.50* | 34 [27, 44]* | 28 [18, 42]* |
| 0.55 | 36 [28, 46] | 29 [19, 44] |
| 0.60 | 37 [29, 48] | 30 [20, 46] |
| 0.65 | 39 [31, 50] | 31 [21, 48] |
| 0.70 | 41 [32, 53] | 33 [22, 50] |
| 0.75 | 43 [34, 55] | 34 [23, 52] |
| 0.80 | 45 [35, 58] | 36 [24, 54] |
| 0.85 | 48 [37, 61] | 37 [25, 56] |
| 0.90 | 50 [39, 64] | 39 [26, 58] |
| 0.95 | 52 [41, 67] | 40 [27, 61] |
| 1.00 | 55 [43, 70] | 42 [28, 63] |
| 1.05 | 58 [45, 74] | 44 [29, 66] |
| 1.10 | 60 [47, 77] | 46 [30, 69] |
| 1.15 | 63 [50, 81] | 48 [32, 72] |
| 1.20 | 66 [52, 85] | 50 [33, 75] |
| 1.25 | 70 [54, 89] | 52 [34, 78] |
| 1.30 | 73 [57, 93] | 54 [36, 81] |
| 1.35† | 77 [60, 98]† | 56 [37, 85] |
| 1.40 | 80 [63, 103] | 59 [39, 88] |
| 1.45 | 84 [66, 108] | 61 [41, 92] |
| 1.50 | 88 [69, 113] | 64 [42, 96] |
| 1.55‡ | 93 [73, 118] | 66 [44, 100]‡ |
| 1.60 | 97 [76, 124] | 69 [46, 104] |
| 1.65 | 102 [80, 130] | 72 [48, 108] |
| 1.70 | 107 [84, 137] | 75 [50, 113] |
| 1.75 | 112 [88, 144] | 78 [52, 118] |
| 1.80 | 118 [92, 151] | 82 [54, 123] |
| 1.85 | 123 [96, 158] | 85 [56, 128] |
| 1.90 | 129 [101, 166] | 89 [59, 134] |
| 1.95 | 136 [106, 174] | 92 [61, 140] |
| ≥2.00 | ≥142 [111, 182] | ≥96 [64, 146] |
Table 2.
Model performances in the validation cohort
| DOAC threshold (ng/mL) | LMWH anti-Xa (IU/mL) | Sensitivity (%) [95% CI] | Specificity (%) [95% CI] | True positive rate* (%) [95% CI] |
|---|---|---|---|---|
| Apixaban | ||||
| <30 | <0.10 | 25 [3.2, 65.1] | 100 [97.6, 100] | 100 [15.8, 100] |
| <50 | <0.64 | 66.7 [48.1, 73.4] | 99.3 [95.9, 100] | 94.7 [74.0, 99.9] |
| <75 | <1.07 | 61.3 [46.0, 83.5] | 100 [96.3, 100] | 100 [90.8, 100] |
| <100 | <1.37 | 63.6 [53.4, 73.1] | 100 [94.1, 100] | 100 [94.3, 100] |
| Rivaroxaban | ||||
| <30 | <0.10 | 6.1 [0.7, 20.2] | 100 [96.6, 100] | 100 [15.8, 100] |
| <50 | <0.71 | 58.9 [45.0, 71.9] | 100 [95.6, 100] | 100 [89.4, 100] |
| <75 | <1.21 | 68.1 [56.0, 78.6] | 100 [94.6, 100] | 100 [92.8, 100] |
| <100 | <1.55 | 75.9 [65.5, 84.4] | 100 [93.0, 100] | 100 [94.6, 100] |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download