Introduction
Materials and Methods
1. Bacterial species and cultivation
2. Susceptibility assay
3. Statistical analysis
Results
1. Antimicrobial activity against A. israelii
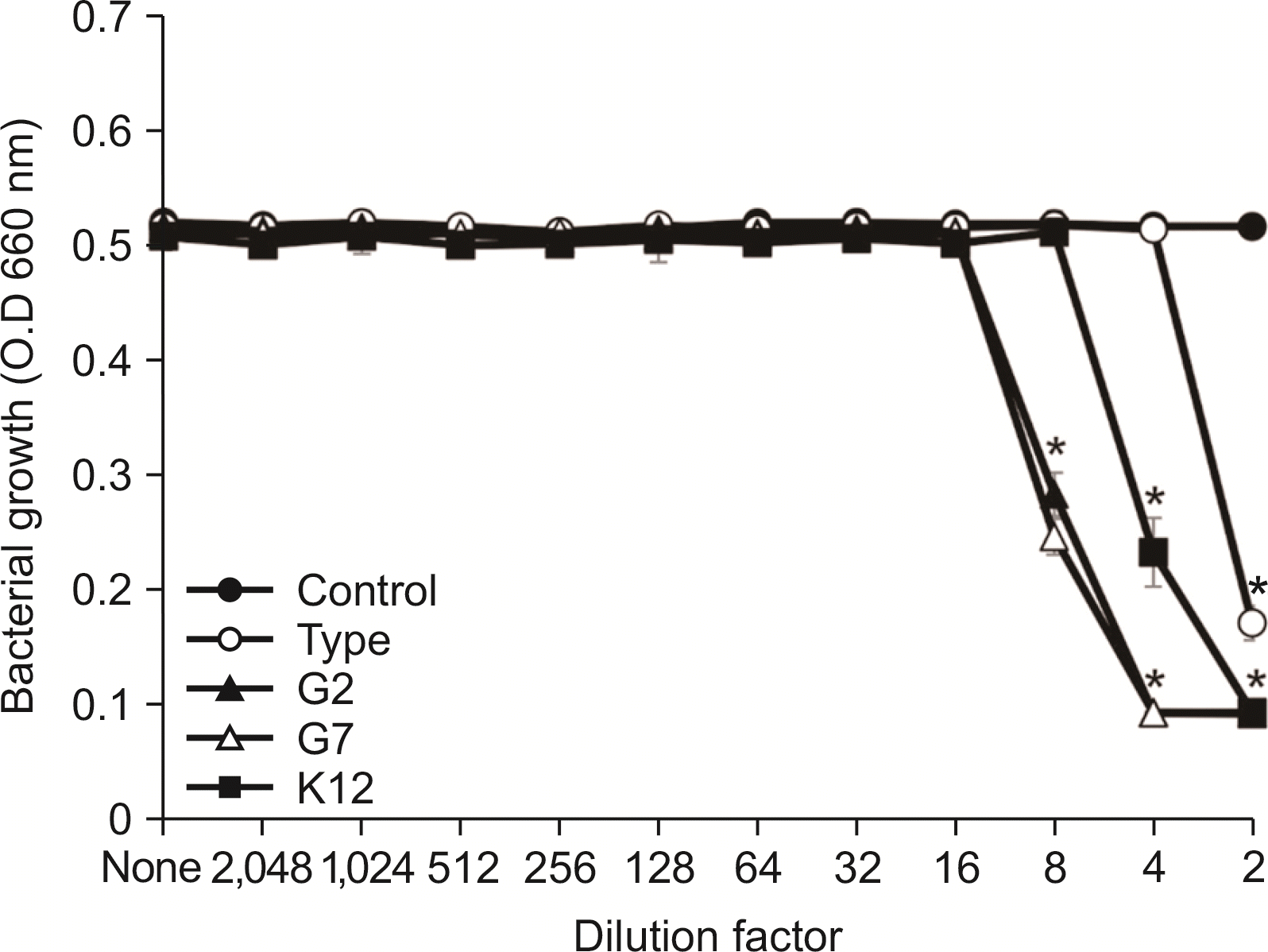 | Fig. 1Susceptibility assay of A. israelii for the spent culture medium of S. salivarius. A. israeliis was cultured in BHI broth and inoculated into TSB. After cultivating overnight, susceptibility test of A. israelii for the SCM of S. salivarius was performed according to the protocol of CLSI. The experiments were performed three times in duplicate and the representative data express mean and standard deviation. *Significance compared to untreated control bacteria (P<0.05). |
2. Antimicrobial activity against A. viscosus
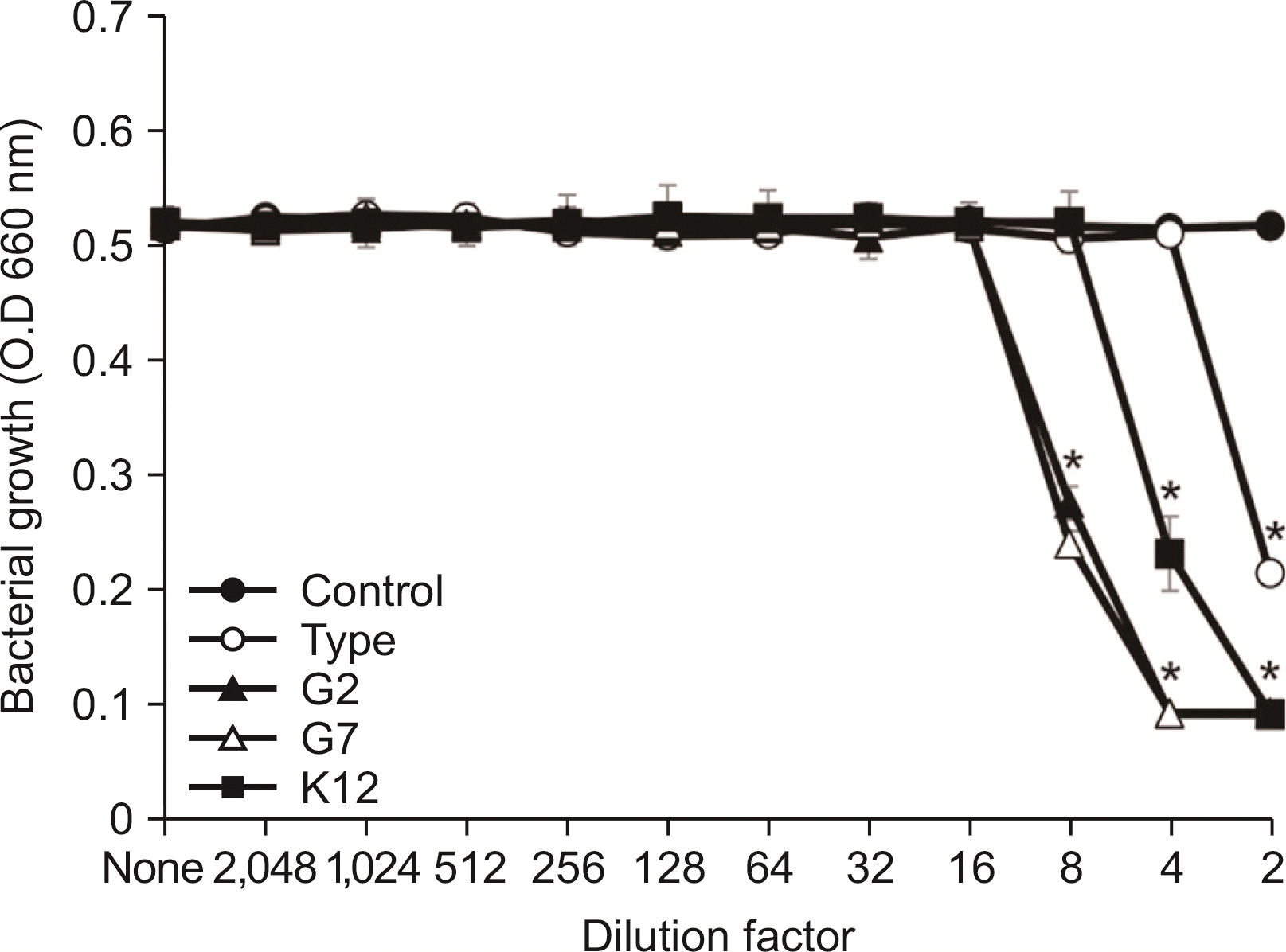 | Fig. 2Antimicrobial activity of the SCM of S. salivarius against A. vicosus. A. viscosus was cultivated in BHI broth and inoculated into TSB. After cultivating overnight, antimicrobial activity of the SCM of S. salivarius against A. viscosus was examined according to the protocol of CLSI. The experiments were performed three times in duplicate and the representative data express mean and standard deviation. *Significance compared to untreated control bacteria (P<0.05). |
3. Antimicrobial activity against E. faecalis
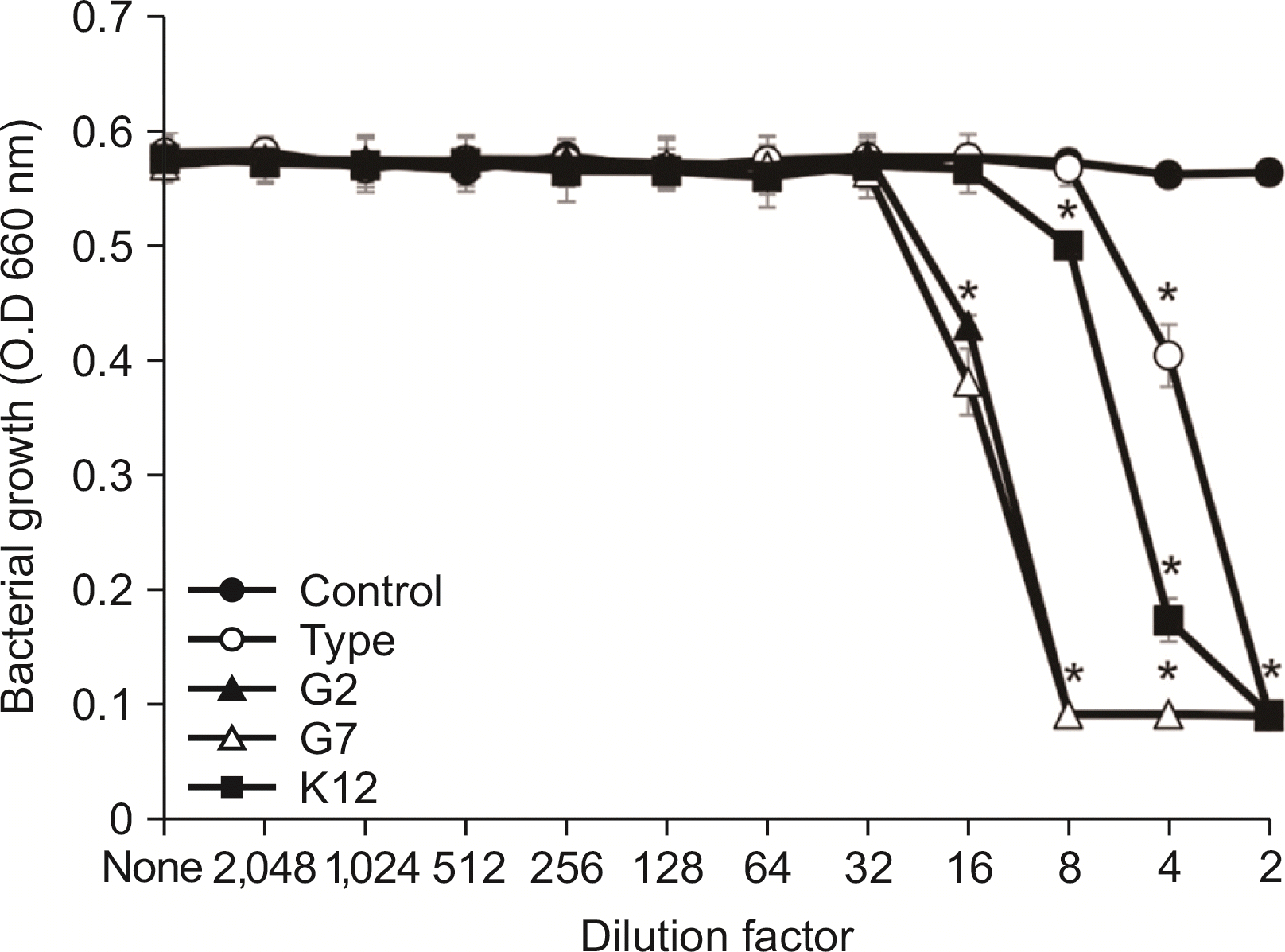 | Fig. 3Susceptibility assay of E. faecalis for the spent culture medium of S. salivarius. E. faecaliss was cultured in BHI broth and inoculated into TSB. After cultivating overnight, susceptibility test of E. faecalis for the SCM of S. salivarius was performed according to the protocol of CLSI. The experiments were performed three times in duplicate and the representative data express mean and standard deviation. *Significance compared to untreated control bacteria (P<0.05). |
4. Antimicrobial activity against S. mutans
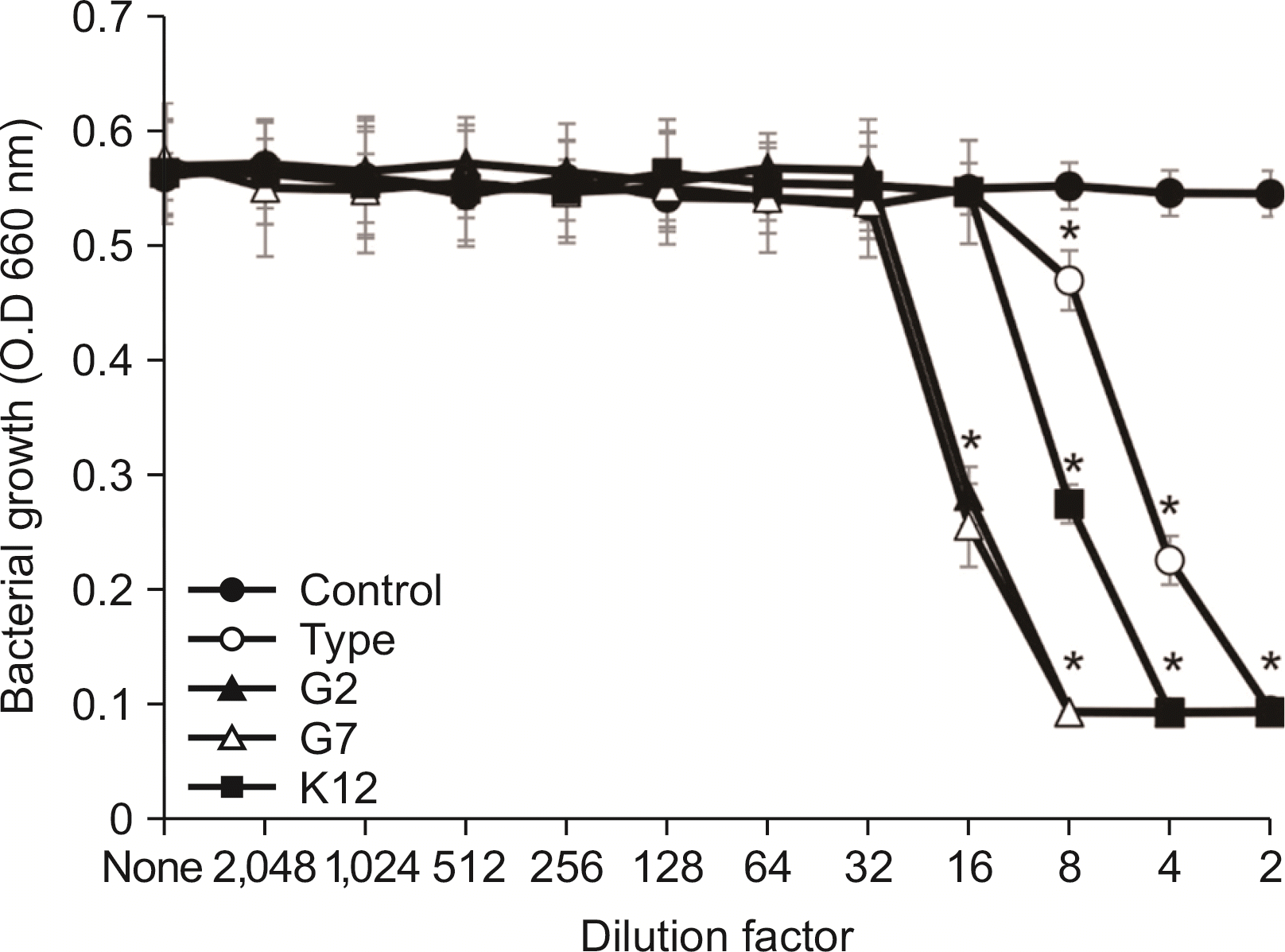 | Fig. 4Antimicrobial activity of the SCM of S. salivarius against S. mutans. S. mutans was cultivated in TSB. After cultivating overnight, antimicrobial activity of the SCM of S. salivarius against S. mutans was examined according to the protocol of CLSI. The experiments were performed three times in duplicate and the representative data express mean and standard deviation. *Significance compared to untreated control bacteria (P<0.05). |
5. Antimicrobial activity against S. sobrinus
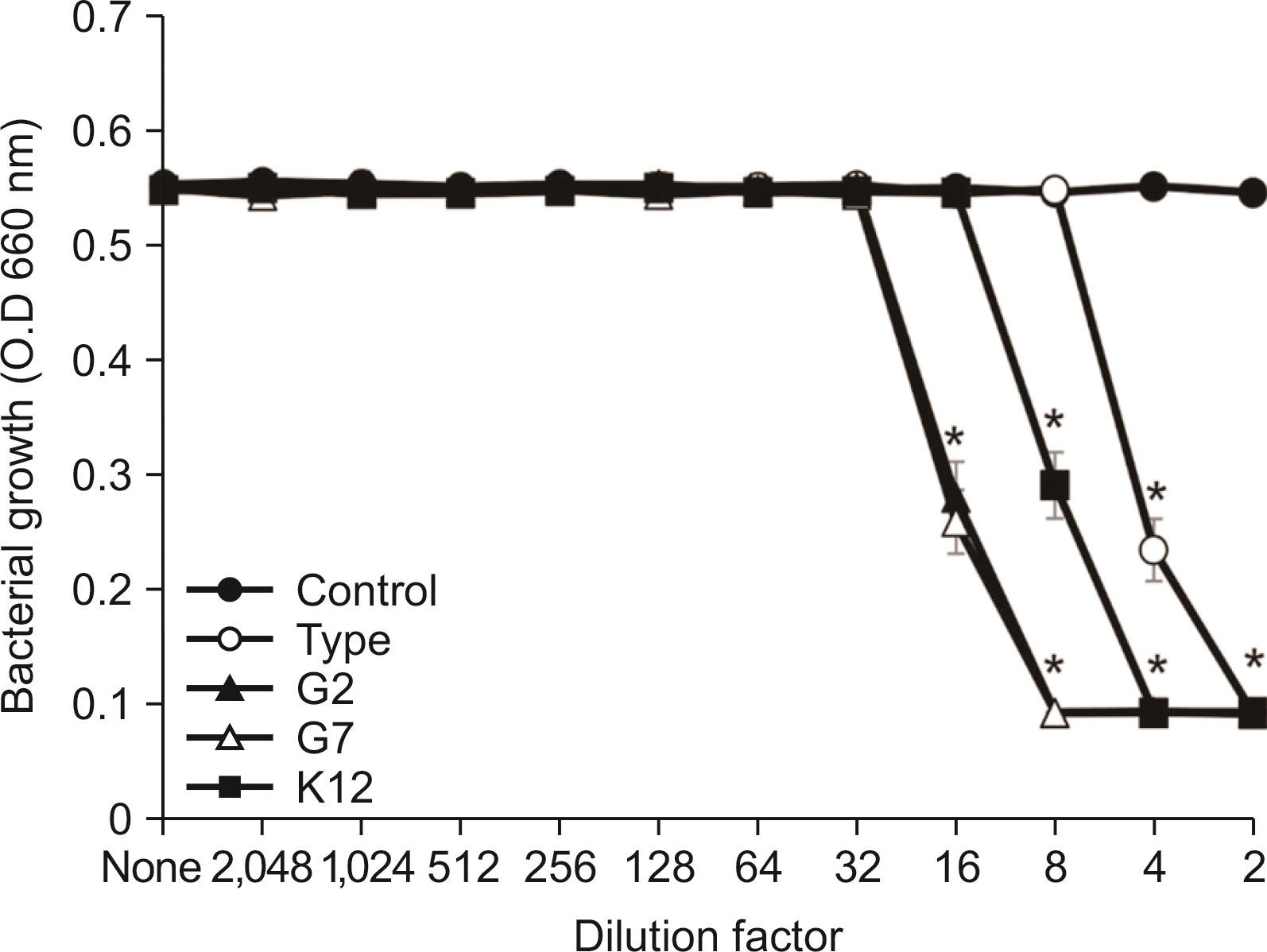 | Fig. 5Susceptibility assay of S. sobrinus for the spent culture medium of S. salivarius. S. sobrinus was cultured in TSB. After cultivating overnight, susceptibility test of S. sobrinus for the SCM of S. salivarius was performed according to the protocol of CLSI. The experiments were performed three times in duplicate and the representative data express mean and standard deviation. *Significance compared to untreated control bacteria (P<0.05). |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download