Abstract
The two main screening tests during pregnancy are those for chromosomal abnormalities and neural tube defects (NTDs). In particular, for NTDs, measurement of maternal serum alpha-fetoprotein (MSAFP) levels early in the second trimester (15–18 weeks of gestation) has been considered the gold standard screening test for the past 4 decades. However, with remarkable technological advancements and the widespread use of ultrasound during those periods, mid-trimester ultrasonography has gradually replaced the role of measuring MSAFP levels as a screening method for NTDs. This change was initiated more about 10 years ago in some countries, which have issued national guidelines to use mid-trimester ultrasonography instead of measuring MSAFP levels as a prenatal screening method for NTDs. However, no significant changes have occurred in Korea, where second-trimester ultrasonography is routinely performed with high-quality equipment. We aimed to provide information regarding the importance of changing the screening method for NTDs from MSAFP measurement to ultrasonography, and to detail methods of implementing mid-trimester ultrasonography for screening purposes. We also share our experience of operating a prenatal diagnostic program for NTDs without using MSAFP for more than 15 years.
During pregnancy, women should be offered screening tests to confirm fetal chromosomal abnormalities such as Down syndrome and fetal neural tube defects (NTDs). As a screening test for NTDs, measurement of maternal serum alphafetoprotein (MSAFP) levels early in the second trimester (15–18 weeks of gestation) has been widely performed as the gold standard screening method for the past 4 decades [1]. However, as prenatal ultrasonography has become more widely performed, along with the remarkable technological advancements in ultrasound equipment, mid-trimester ultrasonography has gradually replaced the role of measuring MSAFP levels as a screening method for NTDs.
Approximately 10 years ago, some countries recommended the use of ultrasonography instead of MSAFP [2–4]. According to the American College of Obstetricians and Gynecologists (ACOG), NTDs could be diagnosed with a high detection rate with two-dimensional ultrasonography alone without any additional laboratory tests from 18–22 weeks of gestation [5]. This was supported by a subsequent study comparing the detection rates of MSAFP measurement and mid-trimester ultrasonography (75% vs. 96%, respectively) [6]. Recently, the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) published guidelines for sonography of the fetal central nervous system (CNS) and recommended multi-planar ultrasonography during the midtrimester, including transventricular, transcerebellar, and transthalamic plane views for the fetal brain and sagittal, axial, and coronal plane views for the fetal spine [7,8]. When intracranial or spinal abnormalities are suspected, a transvaginal approach also is recommended [7]. Recently, the Journal of Obstetrics and Gynecology Canada (JOGC) recommended second-trimester sonography as the primary screening method to detect NTDs [9]. Measurement of MSAFP levels is of limited use, such as in geographic conditions in which midgestational ultrasonography is not feasible or in cases of a high maternal body mass index (≥35 kg/m2) [9]. Although there have been changes in the ACOG and JOGC guidelines regarding screening for NTDs, no significant changes have been made in Korea.
We aimed to provide information regarding the importance of changing the screening method for NTDs from MSAFP measurement to second-trimester ultrasonography and to detail the methods to perform screening ultrasonography. In addition, we share over 15 years of experience from our institution, Chung-Ang University Hospital, which has operated a prenatal diagnostic program for NTDs without MSAFP.
AFP is a glycoprotein that is produced primarily in the yolk sac of the embryo and later in the liver of the fetus. AFP is also found in the gastrointestinal tract, spine, and kidneys of the fetus [10], and was first identified by electrophoresis in 1956 as a protein similar to albumin existing in high concentrations in fetal plasma [11]. In humans, it is encoded by the AFP gene located on chromosome 4 (4q25). AFP permeates into the maternal blood from fetal blood or amniotic fluid. In fetal serum, the highest concentration is 3,000,000 ng/mL at the 9th week (between 10–13 weeks) of gestation, which gradually decreases to approximately 20,000 ng/mL at the end of pregnancy [10,12].
AFP is essentially undetectable (approximately 0.2 ng/mL) in a non-pregnancy state [13], measures approximately 5 ng/mL at 10 weeks of gestation, continues to increase until approximately 32 weeks of gestation, and then gradually decreases until term. The concentration of AFP in amniotic fluid is approximately 1/150th–1/200th that in fetal plasma, and the concentration in maternal serum is approximately 1/100th that in amniotic fluid [14].
Measurement of MSAFP was found to be effective as a screening method for NTDs in the mid-1970s [15,16] and began to spread rapidly in the early 1980s. In 2003, the American Academy of Obstetrics and Gynecology recommended that all pregnant women undergo MSAFP screening in the second trimester of pregnancy [5]. To date, in Korea, almost all local clinics have been measuring MSAFP levels as one axis of the triple test or quadruple test, which is an integrated test to screen for NTDs.
MSAFP measurement is recommended at 16 to 18 weeks and can be performed from 15 to 20 weeks of gestation. The widely accepted criterion of an elevated MSAFP level is 2.5 multiples of the median. Based on this standard, the ability of MSAFP to detect NTDs is reported to be approximately 95% for anencephaly and 65–80% for open spinal NTDs [17].
From the beginning of the ultrasonography era, many obstetric specialists have argued about the superiority of ultrasound compared with MSAFP in diagnosing NTDs [18,19]. However, in general practice, MSAFP has been widely accepted and used as an appropriate screening method for NTDs despite its shortcomings.
There are several disadvantages when using MSAFP, including the various causes of MSAFP elevation, the inappropriate use for closed-type spinal NTDs, high false-positive and-negative rates, and unexplained elevation of MSAFP.
To improve the accuracy of MSAFP measurement, additional information, such as accurate gestational age, maternal weight, race, presence of insulin dependent pre-gestational diabetes, family history of NTDs, and number of fetuses, is required. Thus, there are various potential causes of changes in MSAFP levels including factors that increase and decrease MSAFP levels (Table 1).
There are two types of spinal NTDs (open type and closed type). The closed type accounts for approximately 20% of cases [20]. Measurement of MSAFP levels, in principle, cannot detect closed-type NTDs. However, although tiny closed-type NTDs might be missed, many of them can be detected with ultrasound.
A high false-negative rate of approximately 25% indicates that approximately 25% of open NTDs are missed [17]. Regarding false positives, when the MSAFP level is elevated, amniocentesis is frequently performed to confirm elevations in the amniotic fluid, which increases the medical cost and procedure-related fetal risk accordingly. The vast majority of MSAFP elevations are not associated with amniotic fluid AFP concentration. When MSAFP is elevated, the actual incidence of NTDs is as low as 2–4% [21].
Other than NTDs, elevated MSAFP levels are associated with poor obstetric outcomes [22]. Preterm birth, preeclampsia, fetal growth restriction, and placental pathologies are known to be representative obstetric complications associated with increased MSAFP levels (Table 2) [23]. As ultrasound replaces the role of measuring MSAFP levels in NTD screening, the clinical role of MSAFP measurement is changing to predict placental abnormalities and poor pregnancy outcomes [2,24]. From large-scale studies including over 225,000 screened pregnancies, 20% to 38% of pregnant women with unexplained elevation of MSAFP levels report adverse pregnancy outcomes [25]. Although MSAFP is measured as a component of a quadruple test or an integrated test for aneuploidy screening in Korea, the aneuploidy screening method has gradually changed to a noninvasive prenatal test that measures circulating cell-free DNA. Therefore, MSAFP measurement will gradually become obsolete. Whether MSAFP measurement can be used as a screening method for adverse obstetrical outcomes remains to be determined.
Since the introduction of ultrasonography in the obstetric field, there have been many studies on the effectiveness of ultrasound for the diagnosis of NTDs [18,19]. Although detection of anencephaly is relatively easy, even with early models of ultrasound machines with poor performance, confirmation of spinal NTDs remains within the domain of a few experienced specialists [18,19,26].
As indirect intracranial signs of spinal NTDs were reported [19] along with advancements in ultrasound technology, standard ultrasonography began to show better results than MSAFP measurement in the 1990s [6,27–30]. The RADIUS trial and the Eurofetus study reported 88% and 89% sensitivity, respectively, for NTDs [27,28].
Cranial NTDs, such as anencephaly and encephaloceles, are easily imaged, but spinal NTDs are often difficult to detect due to a supine fetal position, oligohydramnios, or a small defect size. Indirect cranial signs can be helpful to identify spinal NTDs and reduce parental anxiety in high-risk mothers.
These indirect brain signs are caused by the occurrence of the Arnold Chiari malformation due to leakage of cerebrospinal fluid (CSF) through the opening of spinal NTDs. The Arnold Chiari malformation results from a prolapse of the posterior fossa and hindbrain through the foramen magnum. There are three types of Arnold Chiari malformations depending on the morphology and severity. Type I is the mildest form, with downward displacement of the cerebellar tonsils below the foramen magnum. The fourth ventricle remains in the normal position. This is the most common type and usually an incidental finding on a computed tomography or magnetic resonance imaging scan [31,32]. Type II is typically associated with low spinal NTDs and involves herniation of the brainstem, cerebellar tonsils, and vermis [33]. CSF leakage through the opening of spinal NTDs results in a collapse of the fourth ventricle, which leads to a hypoplastic posterior fossa and cerebellar tonsillar herniation. Type III is caused by herniation of the hindbrain into a low occipital or high cervical NTD [34].
In fetuses affected by open spinal NTDs, indirect brain signs are observed in more than 95% of cases [4]. These signs are an abnormal skull shape (lemon sign) (Fig. 1A), an abnormal cerebellum (banana sign) (Fig. 1B), and ventriculomegaly (Fig. 1A) [4,18,19]. Table 3 shows the frequency of indirect signs appearing on mid-trimester ultrasonography [19,35].
The ISUOG recommends evaluation of the spine as a part of the neurosonographic examination [8]. Three types of scanning planes are used to evaluate the fetal spine [8]. Scanning of the axial planes is performed by sweeping the transducer along the entire length of the spine, from the cervical spine to the sacrum, to evaluate the cross-sections of the spine. In this way, three calcifications of the fetal spine can be observed, and the integrity of the posterior skin can be evaluated as well (Fig. 2). The sagittal plane is the most important plane to evaluate the integrity of the fetal spine. In this plane, the ‘S’ shaped normal spinal curve and the integrity of the skin on the back of the spine can be evaluated simultaneously. In this plane, the conus medullaris (CM) (end of the spinal cord) with the filum terminale can be clearly visualized depending on the fetal position (Fig. 3). Almost all spinal NTDs occur in the right middle of the spine; thus, they can be easily detected if the midsagittal plane of the spine is correctly imaged. In fact, for screening purposes, once the entire fetal spine is evaluated clearly in the midsagittal plane, the axial or coronal plane is no longer needed. In the coronal planes, linear calcifications of the fetal spine can be observed. Single line ossification of the vertebral body (Fig. 4A), double line ossification of both pedicles (Fig. 4B), or triple line ossification can be imaged in one coronal plane by adjusting the ultrasound beam (Fig. 4C). Although 3 dinensional (D) ultrasonography can be helpful to evaluate the fetal spine (Fig. 4D), this modality does not provide additional information over 2D ultrasound.
For spinal NTDs, the primary ultrasonographic findings are abnormal posterior vertebral arches and a protruding myelomeningocele sac or indented skin defect over the lesion [36,37]. The axial plane shows divergence of the ossification centers, resulting in a U-shaped vertebra. The sagittal plane shows an irregular posterior skin line (cystic bulging or indented contour) (Fig. 5A, B) [38], and the coronal plane of the affected vertebrae shows additional disrupted parallel ossification lines and skin defects (Fig. 5C, D).
There have been several studies evaluating whether first trimester ultrasonography is effective as a screening test for NTDs [39]. With the advancements in ultrasound technology, first trimester ultrasonography may detect spinal NTDs. To screen for spinal NTDs, cranial signs on first trimester ultrasonography (abnormal intracranial translucency, non-visualization of the cisterna magna, posterior shift of the brainstem toward the occipital bone, and a smaller than expected biparietal diameter) can be useful [40,41]. Spinal ultrasonographic findings show bulging at the posterior contour of the fetal back and an irregular bony spine. However, the detection rate of NTDs with first trimester ultrasonography appears to be much lower than that with second-trimester ultrasonography [39]. Currently, first trimester screening ultrasonography cannot replace second-trimester ultrasonography, but it can be performed by a fetal ultrasound expert for high-risk mothers.
Chung-Ang University Hospital is a tertiary medical center, and we have been performing mid-trimester ultrasonography to detect NTDs without MSAFP measurement for more than 15 years. In the past 8 years (March 2014 to March 2022), there have been 2,956 deliveries, and among them, 1,714 pregnant women received antenatal care at our hospital from the early stages of pregnancy. To screen for NTDs, the primary ultrasound findings that we focus on are the presence of intracranial indirect signs and midsagittal plane views of the spine. There was not a single false-negative case in the past 8 years.
We have also been running a prenatal diagnostic clinic especially for fetal CNS abnormalities for more than 15 years. During the past 8 years, 394 pregnant women with elevated MSAFP levels visited the clinic to have NTDs and other related congenital anomalies ruled out. The characteristics of the study populations are shown in Table 4. After targeted neurosonography, we analyzed the sonographic findings related to MSAFP elevation (Table 5). Thirteen cases demonstrated sonographic findings related to spinal NTDs: seven cases showed definitive sonographic findings of spinal NTDs (indirect brain signs and definitive spinal defects); one case showed a definitive coccygeal defect (3 mm) without indirect brain signs; and five cases were suspected of having a small dimple or indented skin (2–6 mm) in the coccyx region without indirect brain signs. Four fetuses with suspected spinal NTDs were delivered at our hospital. After birth, one small skin dimple, one small filar cyst, and two normal findings were observed. One suspected case of a spinal NTD (1-mm slit-like shadow on sacral region with normal CM imaging and no indirect brain signs) were lost during follow-up. All seven cases with definitive sonographic signs of NTDs could not be followed up. It is assumed that most of the mothers terminated their pregnancies.
We define an unexplained MSAFP elevation (79%) as the absence of congenital abnormalities related to MSAFP elevation, such as NTDs or abdominal wall defects, after targeted ultrasonography. Our data shows that the most common cause related to MSAFP elevation is placental pathology (9%), such as an abnormal placental shape or thickness, placental lake, hematoma formation, and placenta previa. These results are consistent with those of previous studies [24,25,42].
In addition to placental abnormalities, other abnormal ultrasound findings such as fetal growth restriction, polyhydramnios, or oligohydramnios are also associated with MSAFP elevation. All of these are closely related to poor obstetric outcomes.
Based on our clinical experience, sonographically confirmed NTDs occurred in seven of 394 cases of elevated MSAFP levels (2.0%). Unexplained MSAFP elevation occurred in nearly 80% of cases. Even with a high-quality ultrasound machine, sonographic findings of small (less than 6 mm) skin indentations cannot easily rule out closed spinal NTDs.
In Korea, high-performance ultrasound devices are widely available, and most prenatal ultrasounds are performed by obstetricians with sufficient knowledge of obstetric ultrasonography. For the screening and diagnosis of NTDs, early mid-trimester ultrasonography needs to be preferentially selected over MSAFP measurement, which has been used for the past 4 decades. However, it is believed that MSAFP measurement will exist for the time being as part of integrated or sequential tests for chromosomal abnormalities and also can be used for the prognostic index of adverse obstetric outcomes. However, this requires further research and discussion.
When performing mid-trimester ultrasonography to screen for spinal NTDs, it is important to evaluate for indirect brain signs and the entire spine with clear images of the CM in the midsagittal plane. When spinal NTDs are suspected on screening ultrasonography, a second opinion from a prenatal ultrasound specialist is recommended.
Notes
Ethical approval
This study does not require approval of the Institutional Review Board because no patient data is contained in this article. The study was performed in accordance with the principles of the Declaration of Helsinki.
References
1. Driscoll DA, Gross SJ. Screening for fetal aneuploidy and neural tube defects. Genet Med. 2009; 11:818–21.

2. Practice bulletin No. 187: neural tube defects. Obstet Gynecol. 2017; 130:e279–90.
3. Cameron M, Moran P. Prenatal screening and diagnosis of neural tube defects. Prenat Diagn. 2009; 29:402–11.

4. Wilson RD. Prenatal screening, diagnosis, and pregnancy management of fetal neural tube defects. J Obstet Gynaecol Can. 2014; 36:927–39.
5. Cheschier N. ACOG practice bulletin. Neural tube defects. Int J Gynaeco Obstet. 2003; 83:123–33.
6. Norem CT, Schoen EJ, Walton DL, Krieger RC, O’Keefe J, To TT, et al. Routine ultrasonography compared with maternal serum alpha-fetoprotein for neural tube defect screening. Obstet Gynecol. 2005; 106:747–52.

7. Yagel S, Valsky DV. ISUOG practice guidelines (updated): sonographic examination of the fetal central nervous system. Part 1: performance of screening examination and indications for targeted neurosonography. Ultrasound Obstet Gynecol. 2021; 57:173–4.
8. Paladini D, Malinger G, Birnbaum R, Monteagudo A, Pilu G, Salomon LJ, et al. ISUOG practice guidelines (updated): sonographic examination of the fetal central nervous system. Part 2: performance of targeted neurosonography. Ultrasound Obstet Gynecol. 2021; 57:661–71.
9. Douglas Wilson R, Van Mieghem T, Langlois S, Church P. Guideline no. 410: prevention, screening, diagnosis, and pregnancy management for fetal neural tube defects. J Obstet Gynaecol Can. 2021; 43:124–39.

10. Habib ZA. Maternal serum alpha-feto-protein: its value in antenatal diagnosis of genetic disease and in obstetrical-gynaecological care. Acta Obstet Gynecol Scand Suppl. 1977; 61:1–92.

11. Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest. 1956; 8:174.

12. Gitlin D, Boesman M. Serum alpha-fetoprotein, albumin, and gamma-G-globulin in the human conceptus. J Clin Invest. 1966; 45:1826–38.

13. Johansson SG, Kjessler B, Sherman MS, Wahlström J. Alpha-fetoprotein (AFP) levels in maternal serum and amniotic fluid in singleton pregnant women in their 10th-25th week post last menstrual period. Acta Obstet Gynecol Scand Suppl. 1977; 69:20–4.

14. Kim CK, Yang YH. Alpha-fetoprotein values in maternal serum and amniotic fluid for prenatal screening of genetic disorders. Yonsei Med J. 1987; 28:218–27.

15. Leighton PC, Kitau MJ, Chard T, Gordon YB, Leek AE. Levels of alpha-fetoprotein in maternal blood as a screening test for fetal neural-tube defect. Lancet. 1975; 2:1012–5.

16. Wald NJ, Cuckle H, Brock JH, Peto R, Polani PE, Woodford FP. Maternal serum-alpha-fetoprotein measurement in antenatal screening for anencephaly and spina bifida in early pregnancy. Report of U.K. collaborative study on alpha-fetoprotein in relation to neural-tube defects. Lancet. 1977; 1:1323–32.
17. Bradley LA, Palomaki GE, McDowell GA. Technical standards and guidelines: prenatal screening for open neural tube defects. Genet Med. 2005; 7:355–69.
18. Wald N, Cuckle H, Boreham J, Stirrat G. Small biparietal diameter of fetuses with spina bifida: implications for antenatal screening. Br J Obstet Gynaecol. 1980; 87:219–21.

19. Nicolaides KH, Campbell S, Gabbe SG, Guidetti R. Ultrasound screening for spina bifida: cranial and cerebellar signs. Lancet. 1986; 2:72–4.

21. Richards DS, Seeds JW, Katz VL, Lingley LH, Albright SG, Cefalo RC. Elevated maternal serum alpha-fetoprotein with normal ultrasound: is amniocentesis always appropriate? A review of 26,069 screened patients. Obstet Gynecol. 1988; 71:203–7.
22. Puntachai P, Wanapirak C, Sirichotiyakul S, Tongprasert F, Srisupundit K, Luewan S, et al. Associations between pregnancy outcomes and unexplained high and low maternal serum alpha-fetoprotein levels. Arch Gynecol Obstet. 2015; 292:81–5.

23. Gagnon A, Wilson RD. Obstetrical complications associated with abnormal maternal serum markers analytes. J Obstet Gynaecol Can. 2008; 30:918–32.
24. Chandra S, Scott H, Dodds L, Watts C, Blight C, Van den Hof M. Unexplained elevated maternal serum alphafetoprotein and/or human chorionic gonadotropin and the risk of adverse outcomes. Am J Obstet Gynecol. 2003; 189:775–81.
25. Katz VL, Chescheir NC, Cefalo RC. Unexplained elevations of maternal serum alpha-fetoprotein. Obstet Gynecol Surv. 1990; 45:719–26.

26. Hogge WA, Thiagarajah S, Ferguson JE 2nd, Schnatterly PT, Harbert GM Jr. The role of ultrasonography and amniocentesis in the evaluation of pregnancies at risk for neural tube defects. Am J Obstet Gynecol. 1989; 161:520–3.

27. Crane JP, LeFevre ML, Winborn RC, Evans JK, Ewigman BG, Bain RP, et al. A randomized trial of prenatal ultrasonographic screening: impact on the detection, management, and outcome of anomalous fetuses. The RADIUS study group. Am J Obstet Gynecol. 1994; 171:392–9.
28. Grandjean H, Larroque D, Levi S. The performance of routine ultrasonographic screening of pregnancies in the Eurofetus study. Am J Obstet Gynecol. 1999; 181:446–54.

29. Lennon CA, Gray DL. Sensitivity and specificity of ultrasound for the detection of neural tube and ventral wall defects in a high-risk population. Obstet Gynecol. 1999; 94:562–6.

30. Dashe JS, Twickler DM, Santos-Ramos R, McIntire DD, Ramus RM. Alpha-fetoprotein detection of neural tube defects and the impact of standard ultrasound. Am J Obstet Gynecol. 2006; 195:1623–8.

31. Arnautovic A, Splavski B, Boop FA, Arnautovic KI. Pediatric and adult chiari malformation type I surgical series 1965–2013: a review of demographics, operative treatment, and outcomes. J Neurosurg Pediatr. 2015; 15:161–77.

32. Langridge B, Phillips E, Choi D. Chiari malformation type 1: a systematic review of natural history and conservative management. World Neurosurg. 2017; 104:213–19.

33. McLone DG, Knepper PA. The cause of Chiari II malformation: a unified theory. Pediatr Neurosci. 1989; 15:1–12.

34. D’Addario V, Rossi AC, Pinto V, Pintucci A, Di Cagno L. Comparison of six sonographic signs in the prenatal diagnosis of spina bifida. J Perinat Med. 2008; 36:330–4.
35. Kim GJ, Lee ES, Kim DH, Lee SH, Park JD, Park MH. Factors related with intracranial signs in fetuses with open neural tube defect. Obstet Gynecol Sci. 2005; 48:2541–9.
36. Kollias SS, Goldstein RB, Cogen PH, Filly RA. Prenatally detected myelomeningoceles: sonographic accuracy in estimation of the spinal level. Radiology. 1992; 185:109–12.
37. Stephenson SR. Sonographic signs of fetal neural tube and central nervous system defects. J Diagn Med Sonogr. 2003; 19:347–57.

38. Coleman BG, Langer JE, Horii SC. The diagnostic features of spina bifida: the role of ultrasound. Fetal Diagn Ther. 2015; 37:179–96.

39. Fong KW, Toi A, Okun N, Al-Shami E, Menezes RJ. Retrospective review of diagnostic performance of intracranial translucency in detection of open spina bifida at the 11–13-week scan. Ultrasound Obstet Gynecol. 2011; 38:630–4.

40. Mangione R, Dhombres F, Lelong N, Amat S, Atoub F, Friszer S, et al. Screening for fetal spina bifida at the 11–13-week scan using three anatomical features of the posterior brain. Ultrasound Obstet Gynecol. 2013; 42:416–20.
41. Kappou D, Papastefanou I, Pilalis A, Kavalakis I, Kassanos D, Souka AP. Towards detecting open spina bifida in the first trimester: the examination of the posterior brain. Fetal Diagn Ther. 2015; 37:294–300.

42. Williams MA, Hickok DE, Zingheim RW, Luthy DA, Kimelman J, Nyberg DA, et al. Elevated maternal serum alpha-fetoprotein levels and midtrimester placental abnormalities in relation to subsequent adverse pregnancy outcomes. Am J Obstet Gynecol. 1992; 167:1032–7.
Fig. 1
Indirect brain signs of the fetus affected by open spinal neural tube defects. (A) Abnormal skull shape (lemon sign, arrows) and ventriculomegaly at 18 gestational weeks; (B) abnormal cerebellum (banana sign, arrow) at 19 gestational weeks.
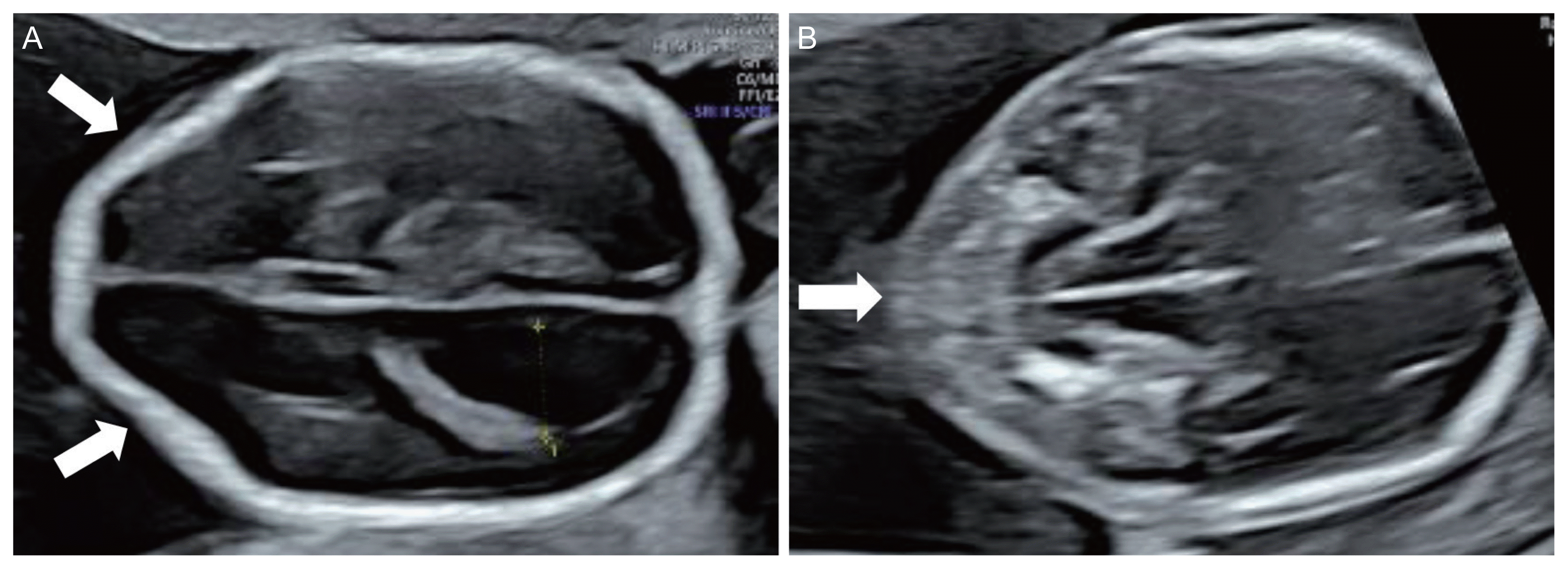
Fig. 2
Axial planes of a normal fetal spine at 25 gestational weeks. (A) Axial plane view of the cervical spine; (B) axial plane view of the thoracic spine; (C) axial plane view of the lumbar spine; (D) axial plane view of the sacrum (arrows: pedicles, arrowhead: vertebral body).
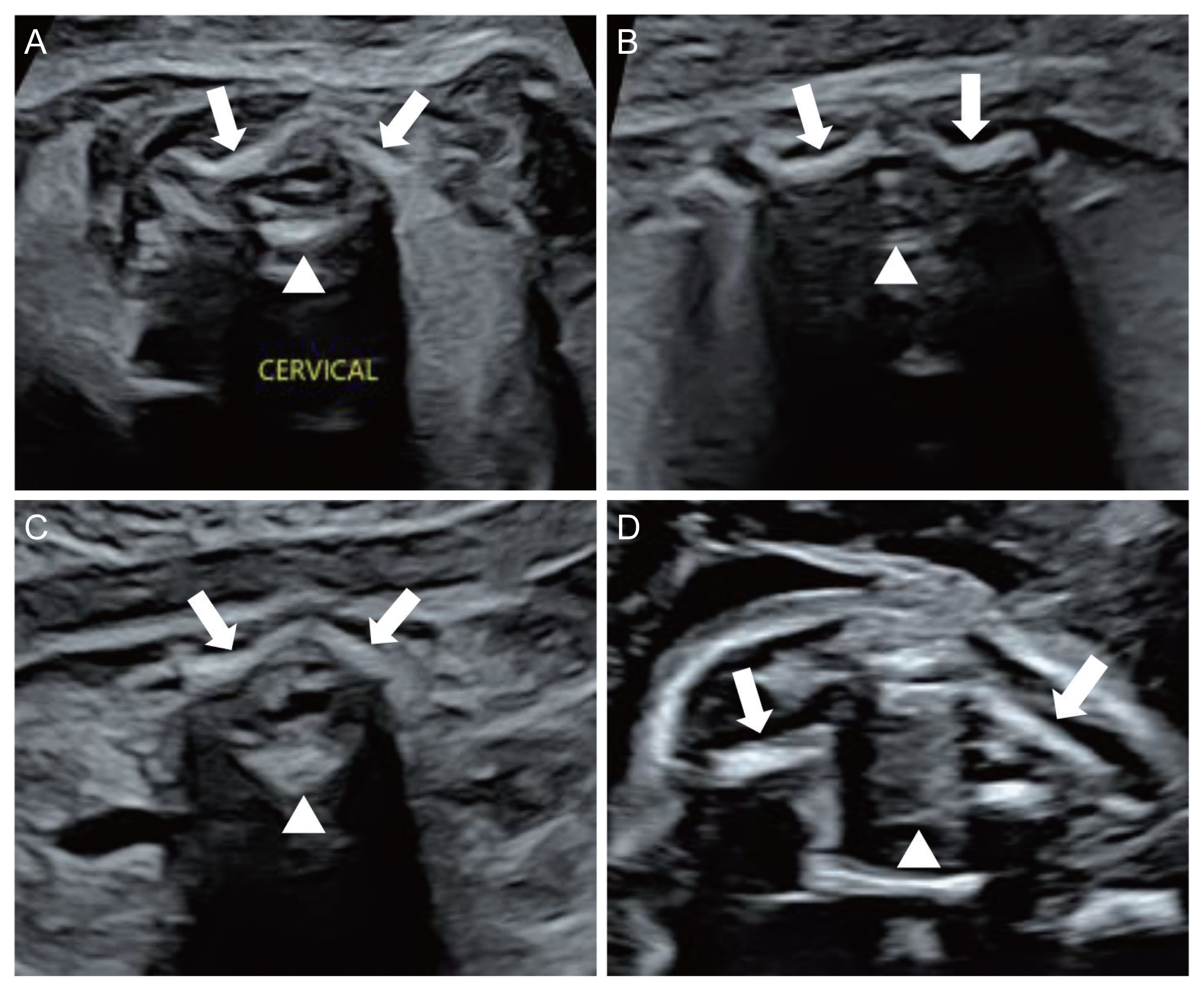
Fig. 3
Sagittal plane view of the normal spine and conus medullaris (CM) at 22 gestational weeks (arrow: filum terminale, arrowhead: central canal).
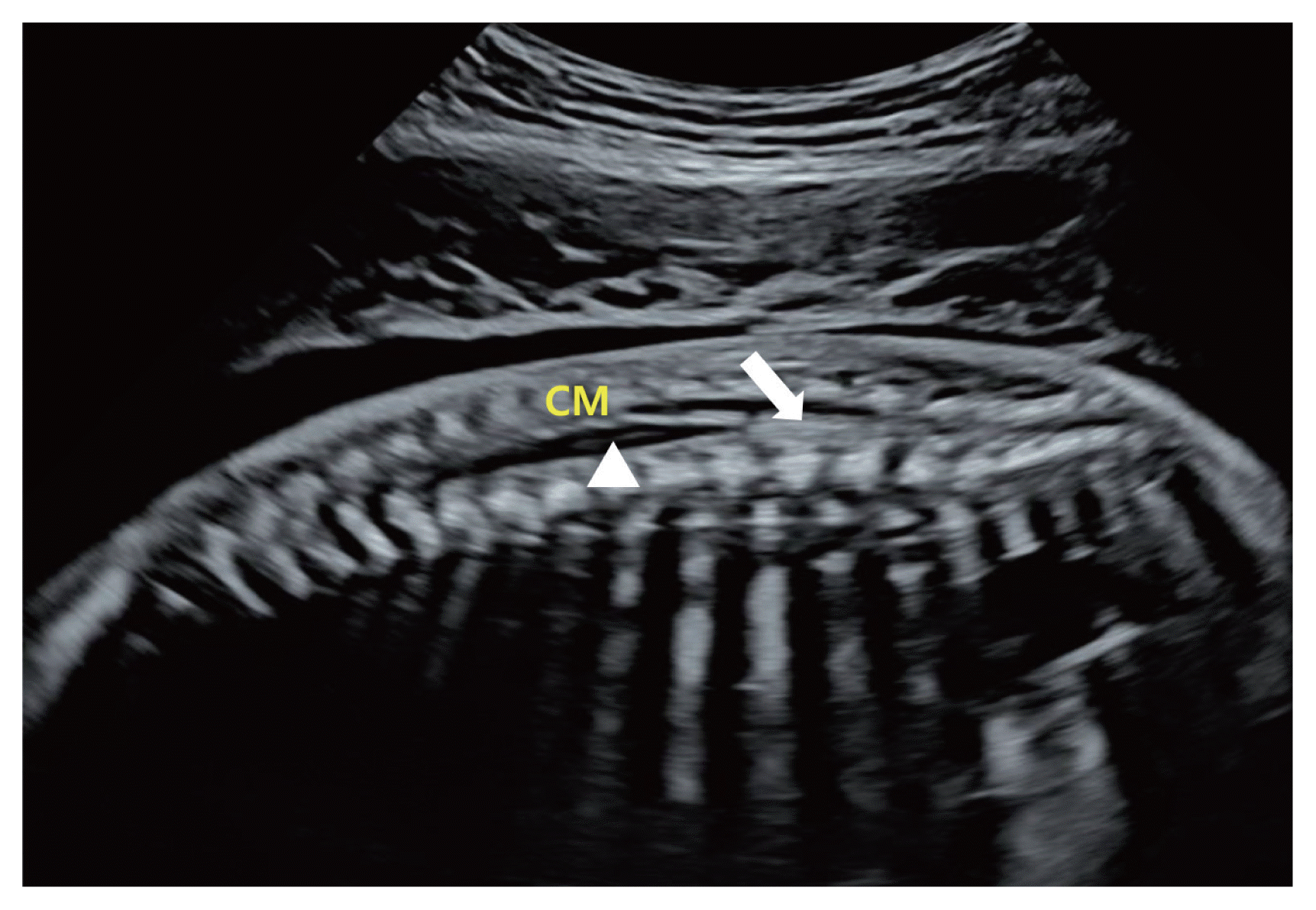
Fig. 4
Coronal plane views of the lumbosacral spine at 25 gestational weeks. (A) Single line (arrowhead: vertebral body) of ossification; (B) double line (arrows: pedicles) of ossification; (C) triple line of ossification (arrows: pedicles, arrowhead: vertebral body). (D) Three-dimensional image of the fetal spine at 21 gestational weeks.
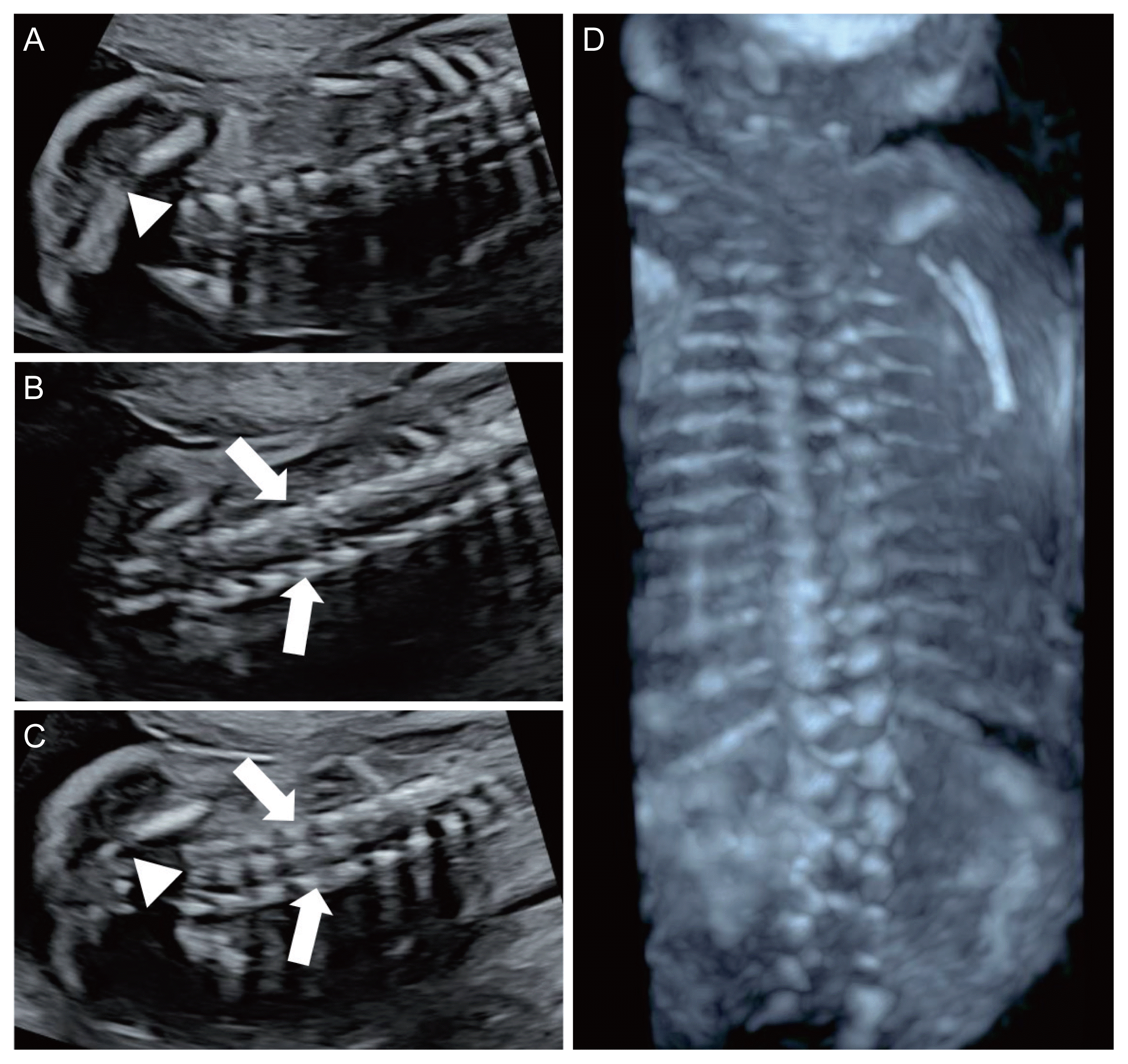
Fig. 5
Ultrasound images of spinal neural tube defects. (A) Protruding cystic mass (arrow) in sagittal plane; (B) indented skin line (arrow) in the sagittal plane; (C) indented skin (arrow) in the coronal plane; (D) ruptured meningomyelocele (arrow) with contour of the lesion (arowheads) in the coronal plane.
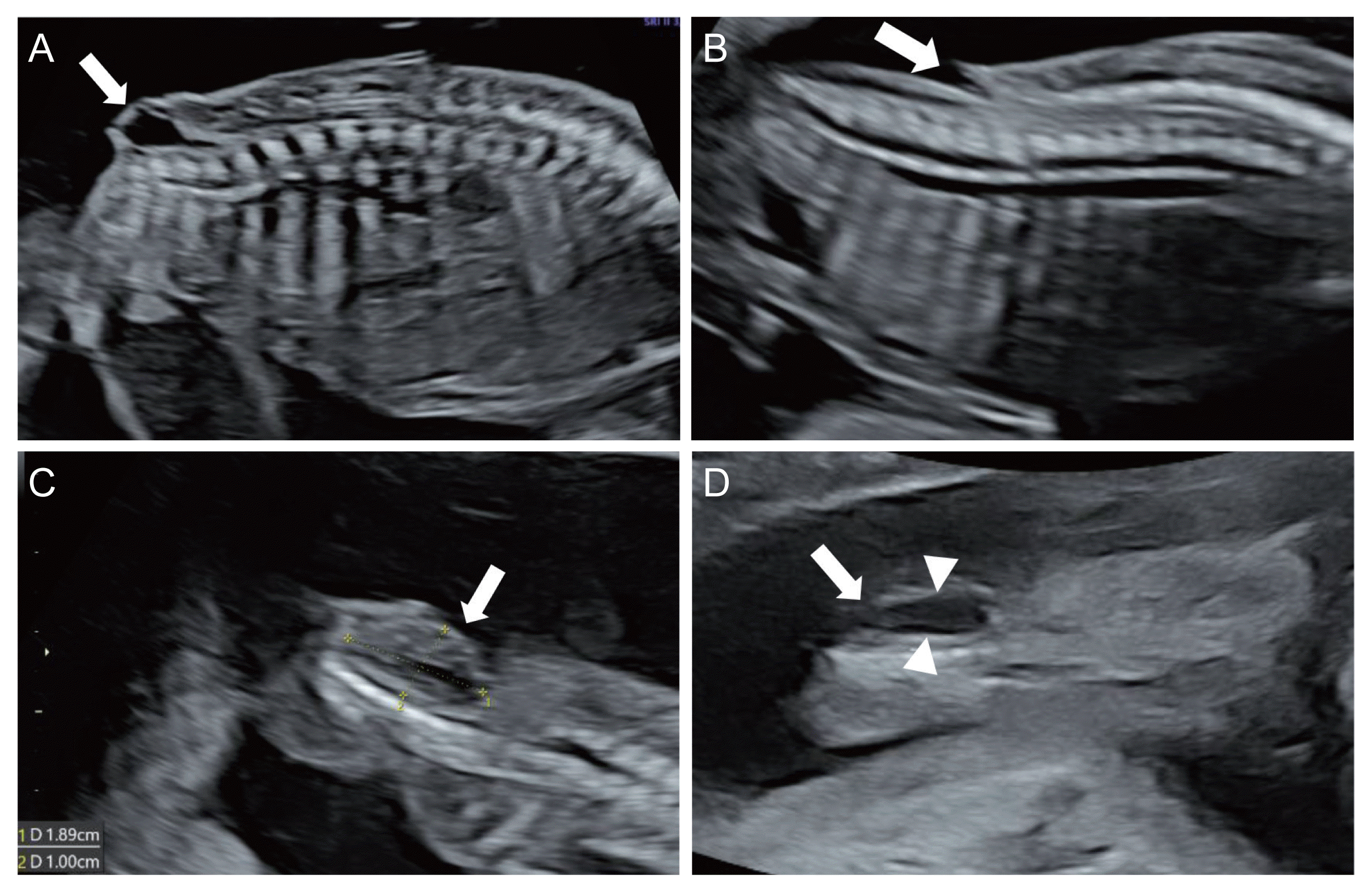
Table 1
Causes of abnormal maternal serum alpha-fetoprotein levels
Table 2
Complications associated with an unexplained elevation of maternal serum alpha-fetoprotein
Adapted from Gagnon and Wilson [23].
Table 3
Frequency of indirect cranial signs of spinal neural tube defects
| Characteristic | Frequency (range reported) |
|---|---|
| Banana sign [19,35] | 50–100 |
| Lemon sign [19,35] | 53–100 |
| Ventriculomegaly [19,35] | 45–89 |
| Small biparietal diameter [19,35] | 53–71a) |
| Small transcerebellar diameter | 72a), 82–96b) |
| Obliteration of the cisterna magna [19,35] | 90–97 |
Table 4
Clinical characteristics of the study population (n=394)
| Characteristic | Value |
|---|---|
| Maternal age (yr) | 32.6±3.9 |
| Maternal BMI | 23.5±3.9 |
| GW at the time of refer | 19.4 (18.3–20.5) |
| MSAFP MoM | 3.0 (2.73–3.5) |
Table 5
Targeted sonographic findings associated with elevated MSAFP levels




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download