1. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006; 24:4539–44.
2. Rudin CM, Ismaila N, Hann CL, Malhotra N, Movsas B, Norris K, et al. Treatment of small-cell lung cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol. 2015; 33:4106–11.
3. Nicholson AG, Chansky K, Crowley J, Beyruti R, Kubota K, Turrisi A, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2016; 11:300–11.
4. Lad T, Piantadosi S, Thomas P, Payne D, Ruckdeschel J, Giaccone G. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest. 1994; 106(6 Suppl):320S–3S.
5. Liao M, Zhao J, Zhou Y. Multimodality therapy of late stage lung cancer. Zhonghua Zhong Liu Za Zhi. 1995; 17:384–6.
6. Inoue M, Miyoshi S, Yasumitsu T, Mori T, Iuchi K, Maeda H, et al. Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg. 2000; 70:1615–9.
7. Brock MV, Hooker CM, Syphard JE, Westra W, Xu L, Alberg AJ, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J Thorac Cardiovasc Surg. 2005; 129:64–72.
8. Lim E, Belcher E, Yap YK, Nicholson AG, Goldstraw P. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol. 2008; 3:1267–71.
9. Yang CJ, Chan DY, Shah SA, Yerokun BA, Wang XF, D’Amico TA, et al. Long-term survival after surgery compared with concurrent chemoradiation for node-negative small cell lung cancer. Ann Surg. 2018; 268:1105–12.
10. Schreiber D, Rineer J, Weedon J, Vongtama D, Wortham A, Kim A, et al. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: should its role be re-evaluated? Cancer. 2010; 116:1350–7.
11. Wakeam E, Acuna SA, Leighl NB, Giuliani ME, Finlayson SRG, Varghese TK, et al. Surgery versus chemotherapy and radiotherapy for early and locally advanced small cell lung cancer: a propensity-matched analysis of survival. Lung Cancer. 2017; 109:78–88.

13. Ou SH, Zell JA. The applicability of the proposed IASLC staging revisions to small cell lung cancer (SCLC) with comparison to the current UICC 6th TNM edition. J Thorac Oncol. 2009; 4:300–10.

14. Vallieres E, Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2009; 4:1049–59.
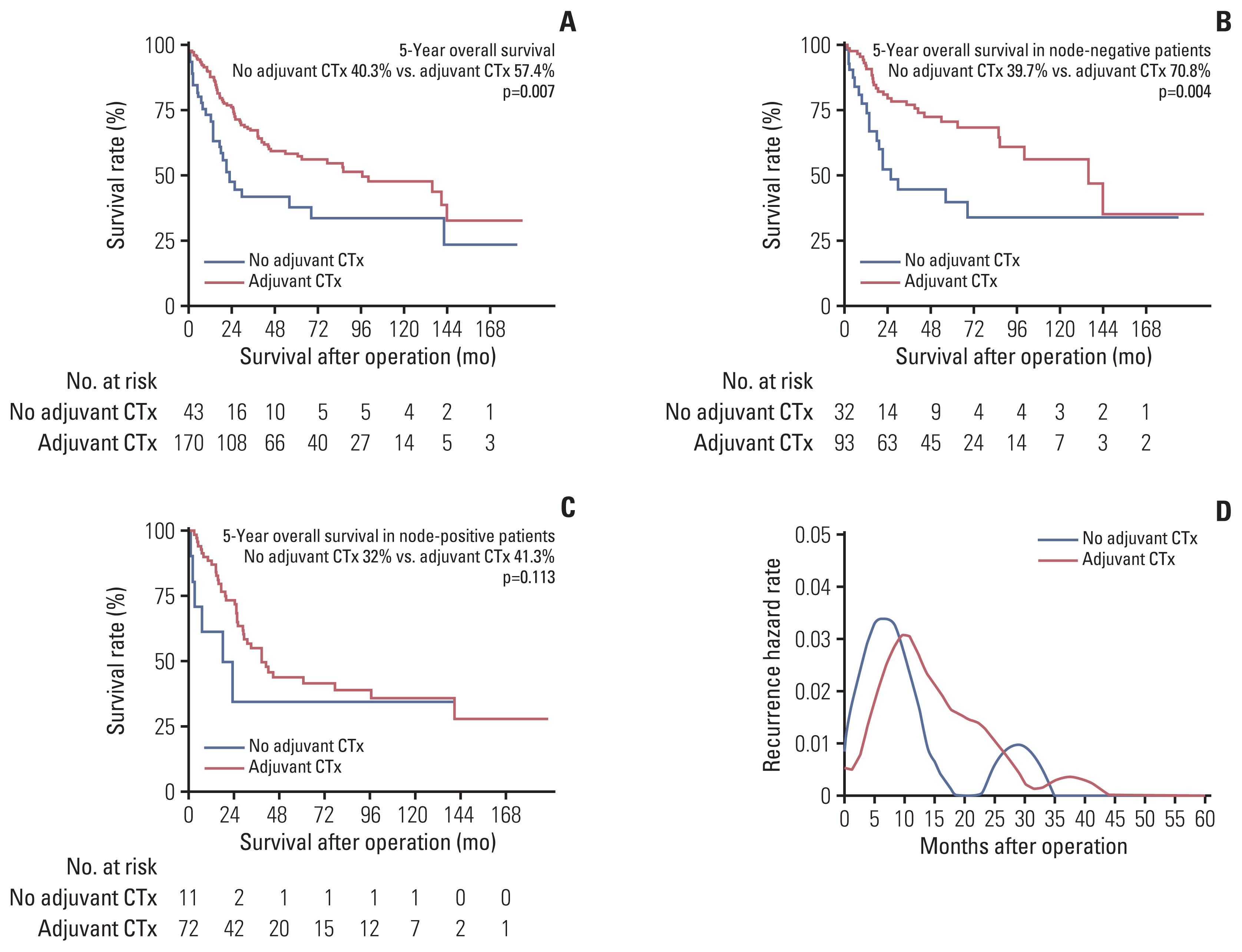
15. Yang CF, Chan DY, Speicher PJ, Gulack BC, Wang X, Hartwig MG, et al. Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol. 2016; 34:1057–64.
16. Komaki R, Khalid N, Langer CJ, Kong FM, Owen JB, Crozier CL, et al. Penetration of recommended procedures for lung cancer staging and management in the United States over 10 years: a quality research in radiation oncology survey. Int J Radiat Oncol Biol Phys. 2013; 85:1082–9.

17. Bischof M, Debus J, Herfarth K, Muley T, Kappes J, Storz K, et al. Surgery and chemotherapy for small cell lung cancer in stages I–II with or without radiotherapy. Strahlenther Onkol. 2007; 183:679–84.
18. Zhou N, Bott M, Park BJ, Vallieres E, Wilshire CL, Yasufuku K, et al. Predictors of survival following surgical resection of limited-stage small cell lung cancer. J Thorac Cardiovasc Surg. 2021; 161:760–71.
19. Muller HG, Wang JL. Hazard rate estimation under random censoring with varying kernels and bandwidths. Biometrics. 1994; 50:61–76.
20. Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013; 143(5 Suppl):e400S–19S.
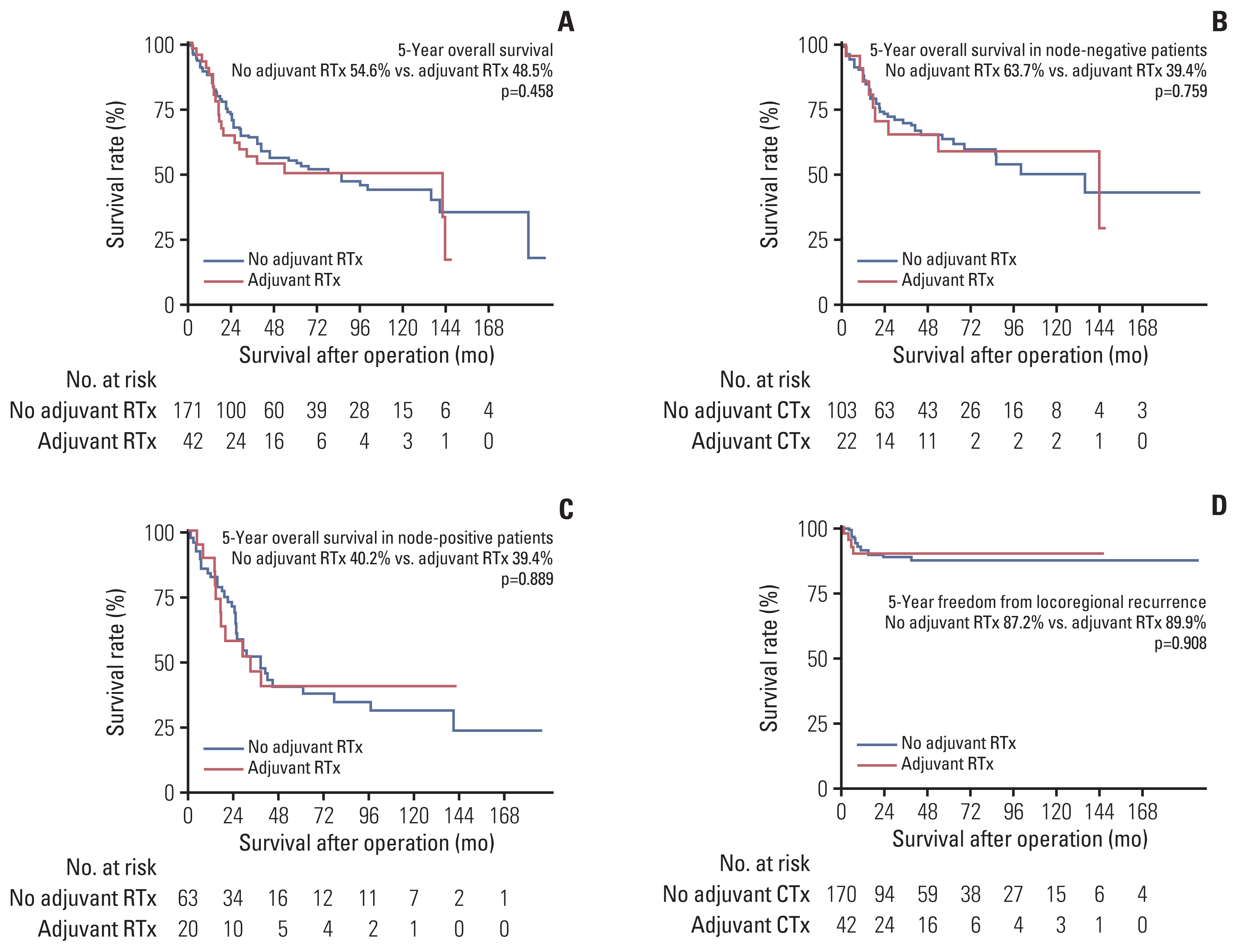
21. Wakeam E, Giuliani M, Leighl NB, Finlayson SR, Varghese TK, Darling GE. Indications for adjuvant mediastinal radiotherapy in surgically resected small cell lung cancer. Ann Thorac Surg. 2017; 103:1647–53.
22. Combs SE, Hancock JG, Boffa DJ, Decker RH, Detterbeck FC, Kim AW. Bolstering the case for lobectomy in stages I, II, and IIIA small-cell lung cancer using the National Cancer Data Base. J Thorac Oncol. 2015; 10:316–23.
23. Park BJ, Cho JH, Lee JH, Shin S, Kim HK, Choi YS, et al. Temporal and regional distribution of initial recurrence site in completely resected N1-stage II lung adenocarcinoma: the effect of postoperative adjuvant chemotherapy. Lung Cancer. 2018; 117:7–13.
24. Lee J, Kim HK, Park BJ, Cho JH, Choi YS, Zo JI, et al. Recurrence dynamics after trimodality therapy (neoadjuvant concurrent chemoradiotherapy and surgery) in patients with stage IIIA (N2) lung cancer. Lung Cancer. 2018; 115:89–96.