Abstract
Purpose
Materials and Methods
Results
Electronic Supplementary Material
Notes
Ethical Statement
This study was reviewed and approved by the Institutional Review Board (IRB approval number: 2019-1476) of the Asan Medical Center (Seoul, South Korea) and Samsung Medical Center (Seoul, South Korea). The requirement of informed consent was waived by the IRBs because of the study’s retrospective nature.
Author Contributions
Conceived and designed the analysis: Suh C.
Collected the data: Kang S, Lee K, Kang EH, Park JS, Lee YS, Park CS, Go H, Huh J, Ryu JS, Lee SW, Kim SJ, Kim WS, Yoon SE, Ko YH, Suh C.
Contributed data or analysis tools: Kang S, Cho H, Lee K, Kang EH, Park JS, Lee YS, Park CS, Go H, Huh J, Ryu JS, Lee SW, Kim SJ, Kim WS, Yoon SE, Ko YH, Suh C.
Performed the analysis: Kang S, Cho H, Suh C.
Wrote the paper: Kang S, Cho H, Suh C.
Review the manuscript: Lee K, Kang EH, Park JS, Lee YS, Park CS, Go H, Ryu JS, Lee SW, Kim SJ, Kim WS, Yoon SE, Ko YH.
References
Fig. 1
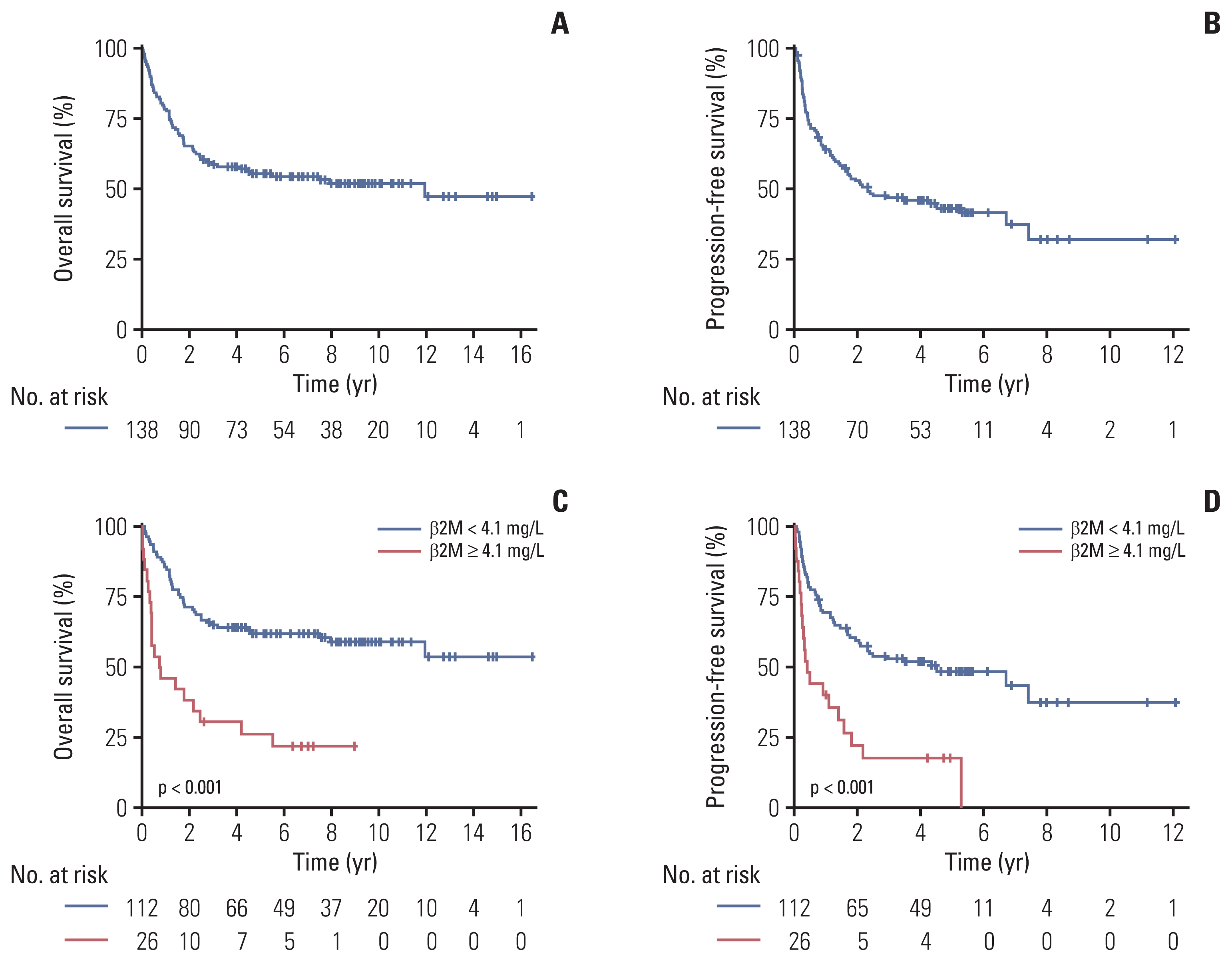
Fig. 2
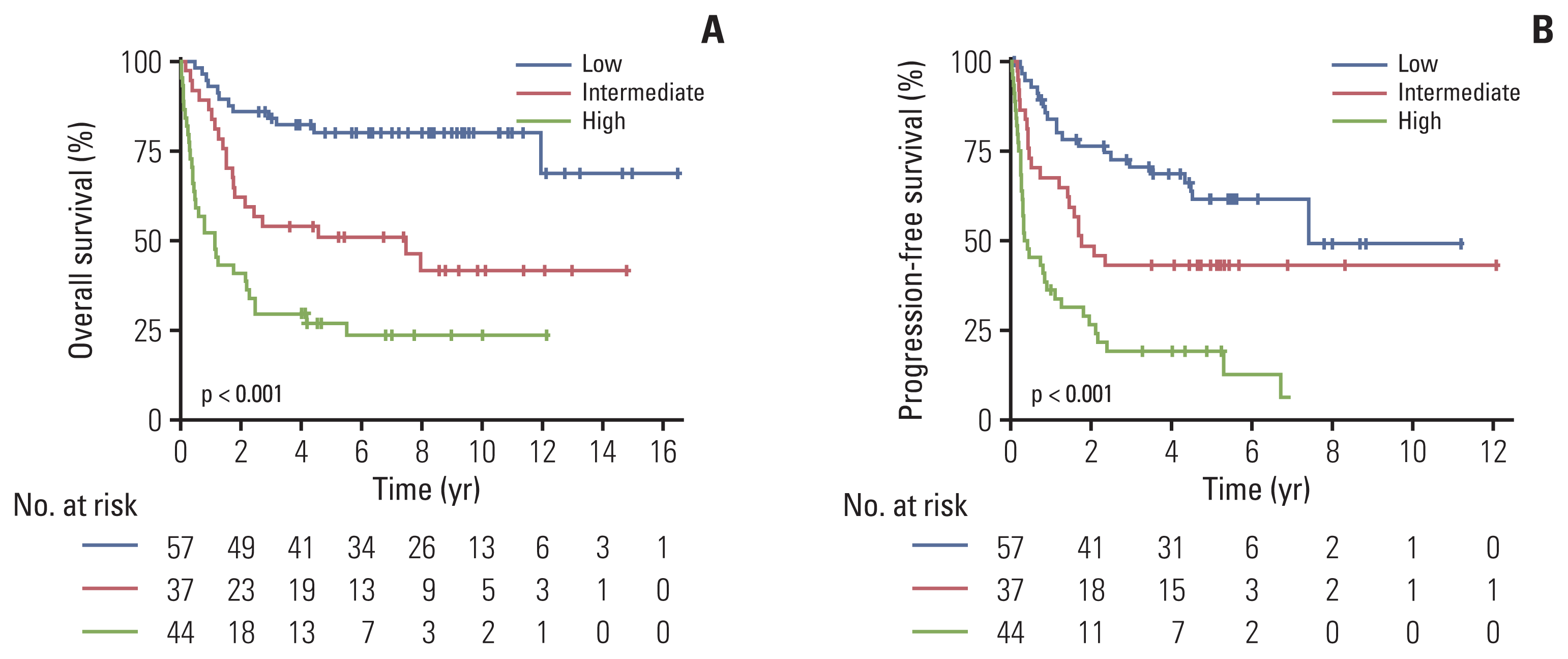
Fig. 3
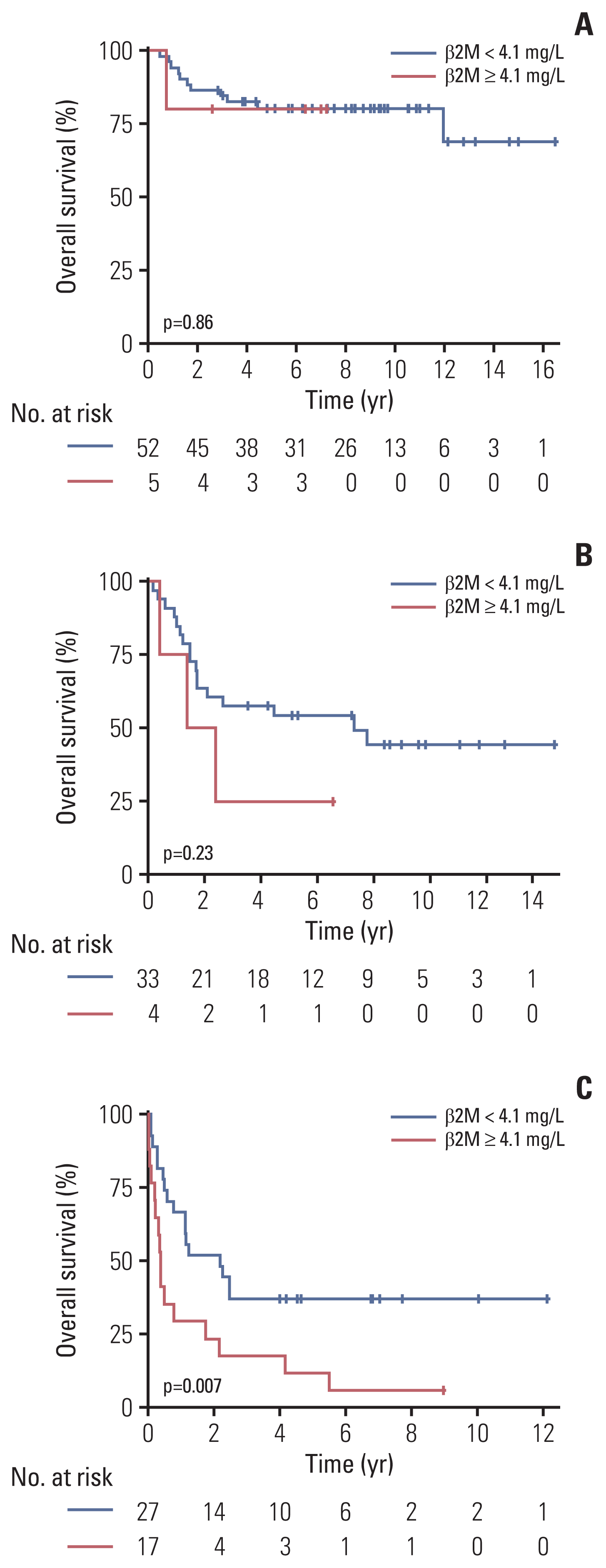
Fig. 4
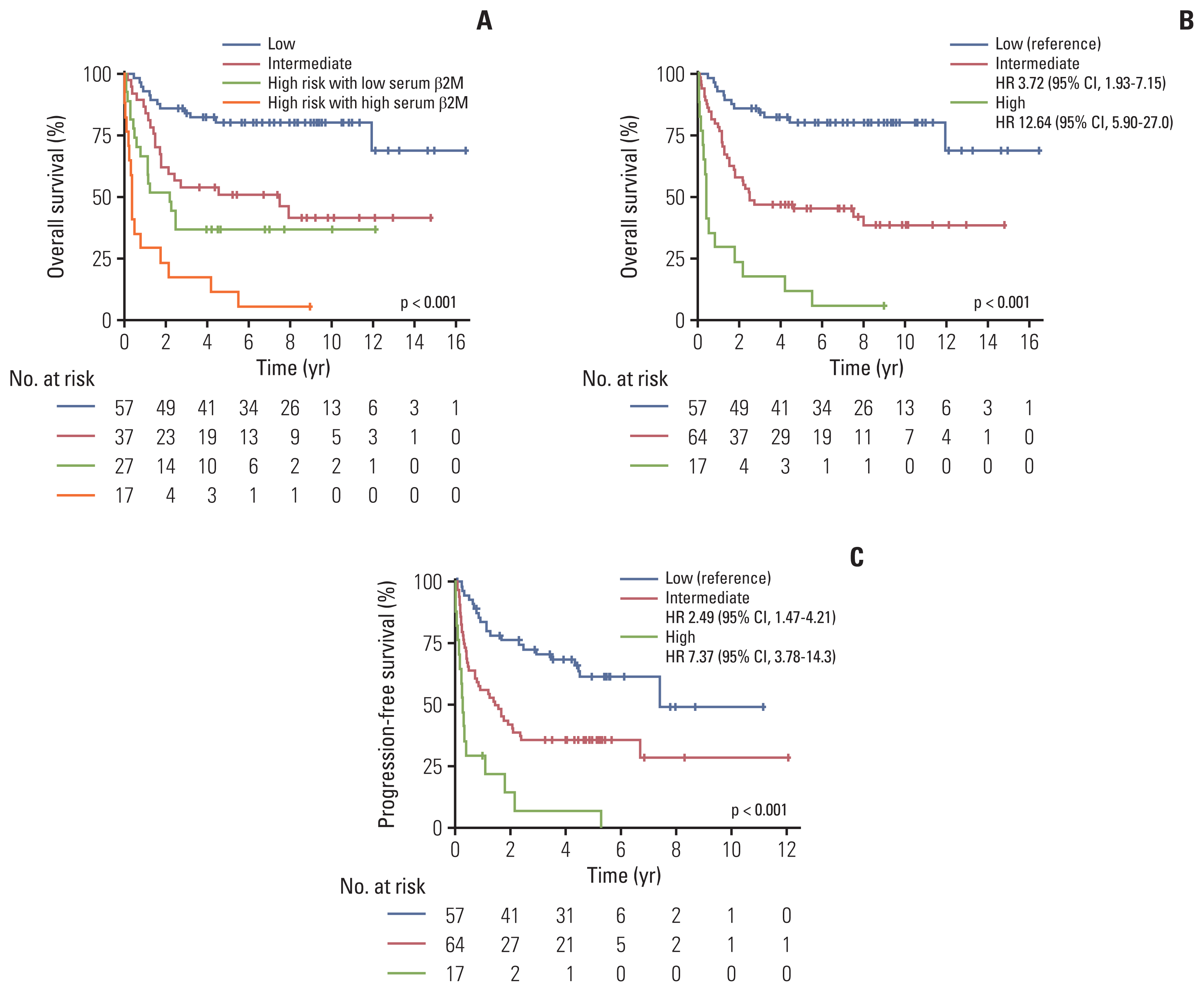
Fig. 5
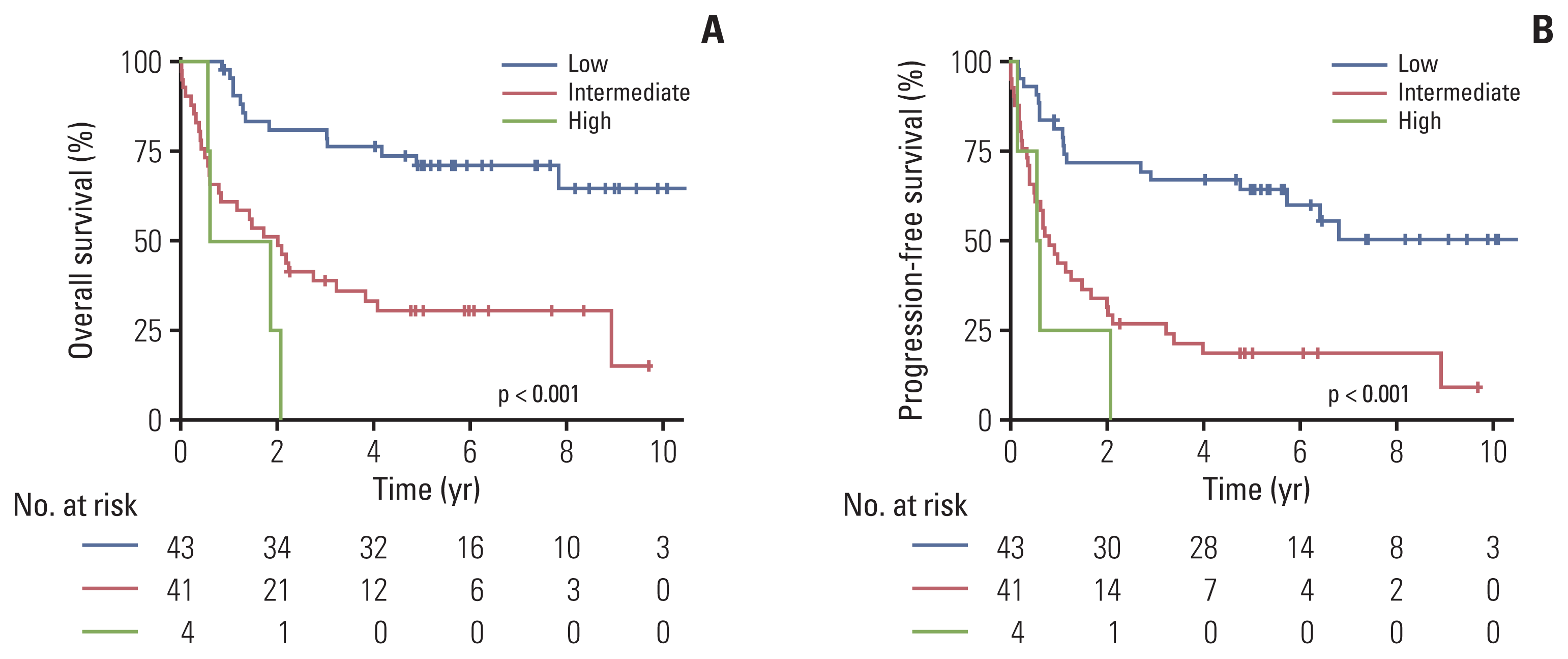
Table 1
| Total (n=138) | Low β2M (< 4.1) (n=112) | High β2M (≥ 4.1) (n=26) | p-value | |
|---|---|---|---|---|
| Age (yr) | ||||
| ≤ 60 | 94 (68.1) | 77 (68.8) | 17 (65.4) | 0.740 |
| > 60 | 44 (31.9) | 35 (31.2) | 9 (34.6) | |
| Median (range) | 53.5 (19–80) | 53.5 (19–80) | 54 (25–79) | 0.682 |
| Sex | ||||
| Male | 86 (62.3) | 71 (63.4) | 15 (57.7) | 0.589 |
| Female | 52 (37.7) | 41 (36.6) | 11 (42.3) | |
| ECOG PS | ||||
| 0 or 1 | 129 (93.5) | 109 (97.3) | 20 (76.9) | 0.001 |
| ≥ 2 | 9 (6.5) | 3 (2.7) | 6 (23.1) | |
| Ann Arbor stage | ||||
| I or II | 93 (67.4) | 83 (74.1) | 10 (38.5) | 0.001 |
| III or IV | 45 (32.6) | 29 (25.9) | 16 (61.5) | |
| Lymph node involvement | ||||
| None/Regional | 99 (71.7) | 90 (80.4) | 9 (34.6) | < 0.001 |
| Distant | 39 (28.3) | 22 (19.6) | 17 (65.4) | |
| Non-nasal type | ||||
| No | 114 (82.6) | 96 (85.7) | 18 (69.2) | 0.080 |
| Yes | 24 (17.4) | 16 (14.3) | 8 (30.8) | |
| Bone marrow involvement | ||||
| No | 123 (89.8) | 104 (93.7) | 19 (73.1) | 0.006 |
| Yes | 14 (10.2) | 7 (6.3) | 7 (26.9) | |
| Serum LDH | ||||
| Normal | 78 (56.9) | 76 (68.5) | 2 (7.7) | < 0.001 |
| Elevated | 59 (43.1) | 35 (31.5) | 24 (92.3) | |
| Serum creatinine | ||||
| Normal (< 1.5 mg/dL) | 137 (99.2) | 112 (100) | 25 (96.1) | 0.188 |
| Elevated (≥ 1.5 mg/dL) | 1 (0.7) | 0 | 1 (3.8) | |
| Epstein-Barr virus DNA in blood | ||||
| Detectable | 71 (51.4) | 49 (43.7) | 22 (84.6) | < 0.001 |
| Non-detectable | 61 (44.2) | 58 (51.7) | 3 (11.5) | |
| Unknown | 6 (4.3) | 5 (4.5) | 1 (3.8) | |
| Primary treatment | ||||
| Chemotherapy alone | 56 (40.6) | 37 (33.0) | 19 (73.1) | < 0.001 |
| CCRT alone | 11 (7.97) | 10 (8.9) | 1 (3.8) | |
| CCRT followed by chemotherapy | 71 (51.4) | 65 (58.0) | 6 (23.1) | |
| PINK risk group | ||||
| PINK low | 57 (41.3) | 52 (46.4) | 5 (19.2) | < 0.001 |
| PINK intermediate | 37 (26.8) | 33 (29.5) | 4 (15.4) | |
| PINK high | 44 (31.9) | 27 (24.1) | 17 (65.4) | |
| Extranodal involvement | ||||
| 0 or 1 | 92 (66.6) | 82 (74.5) | 10 (35.7) | < 0.001 |
| ≥ 2 | 46 (33.3) | 28 (25.5) | 18 (64.3) | |
| Chemotherapy regimen a) | ||||
| SMILE | 35 (25.3) | 22 (19.6) | 13 (50.0) | 0.012 |
| VIDL | 67 (48.5) | 59 (52.7) | 8 (30.8) | |
| VIPD | 15 (10.8) | 13 (11.6) | 2 (7.7) | |
| MIDLE | 4 (2.89) | 4 (3.6) | 0 | |
| IMEP | 3 (2.1) | 3 (2.7) | 0 | |
| DeVIC | 1 (0.72) | 1 (0.9) | 0 | |
| DHAP | 1 (0.72) | 0 | 1 (3.8) | |
| ESHAP | 1 (0.72) | 0 | 1 (3.8) | |
Values ae presented as number (%) or median (range). β2M, serum β-2 microglobulin; CCRT, concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; PINK, Prognostic Index for Natural Killer Lymphoma; PS, performance status.
a) Chemotherapy regimen abbreviations: SMILE, corticosteroid, methotrexate, ifosfamide, L-asparaginase, and etoposide; VIDL, etoposide, ifosfamide, dexamethasone, and L-asparaginase; VIPD, etoposide, ifosfamide, cisplatin, and dexamethasone; MIDLE, methotrexate, ifosfamide, dexamethasone, L-asparaginase, and etoposide; IMEP, ifosfamide, methotrexate, etoposide, and prednisolone; DeVIC, dexamethasone, etoposide, ifosfamide, and carboplatin; DHAP, dexamethasone, cisplatin, and cytarabine; ESAHP, etoposide, corticosteroid, cytarabine, and cisplatin.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download