Abstract
Purpose
Materials and Methods
Results
Notes
Ethical Statement
Written informed consent was obtained from participants in the Korean National Cancer Screening Program (KNCSP) for the collection of screening results; the requirement for informed consent for the current study was waived owing to the use of de-identified data. With permission from the Ministry of Health and Welfare, the investigators used data maintained and de-identified by the NHIS. This study was approved by the Institutional Review Board of the National Cancer Center, Korea (IRB No. NCCNCS08129).
Author Contributions
Conceived and designed the analysis: Choi E, Suh M, Jung SY, Park S, Jun JK, Choi KS.
Contributed data or analysis tools: Choi E, Suh M, Jung KW, Jun JK, Choi KS.
Performed the analysis: Choi E.
Wrote the paper: Choi E, Choi KS.
Writing - original draft: Choi E.
Review and editing: Choi E, Suh M, Jung SY, Jung KW, Park S, Jun JK, Choi KS.
Supervision: Choi KS.
Acknowledgments
References
Fig. 1
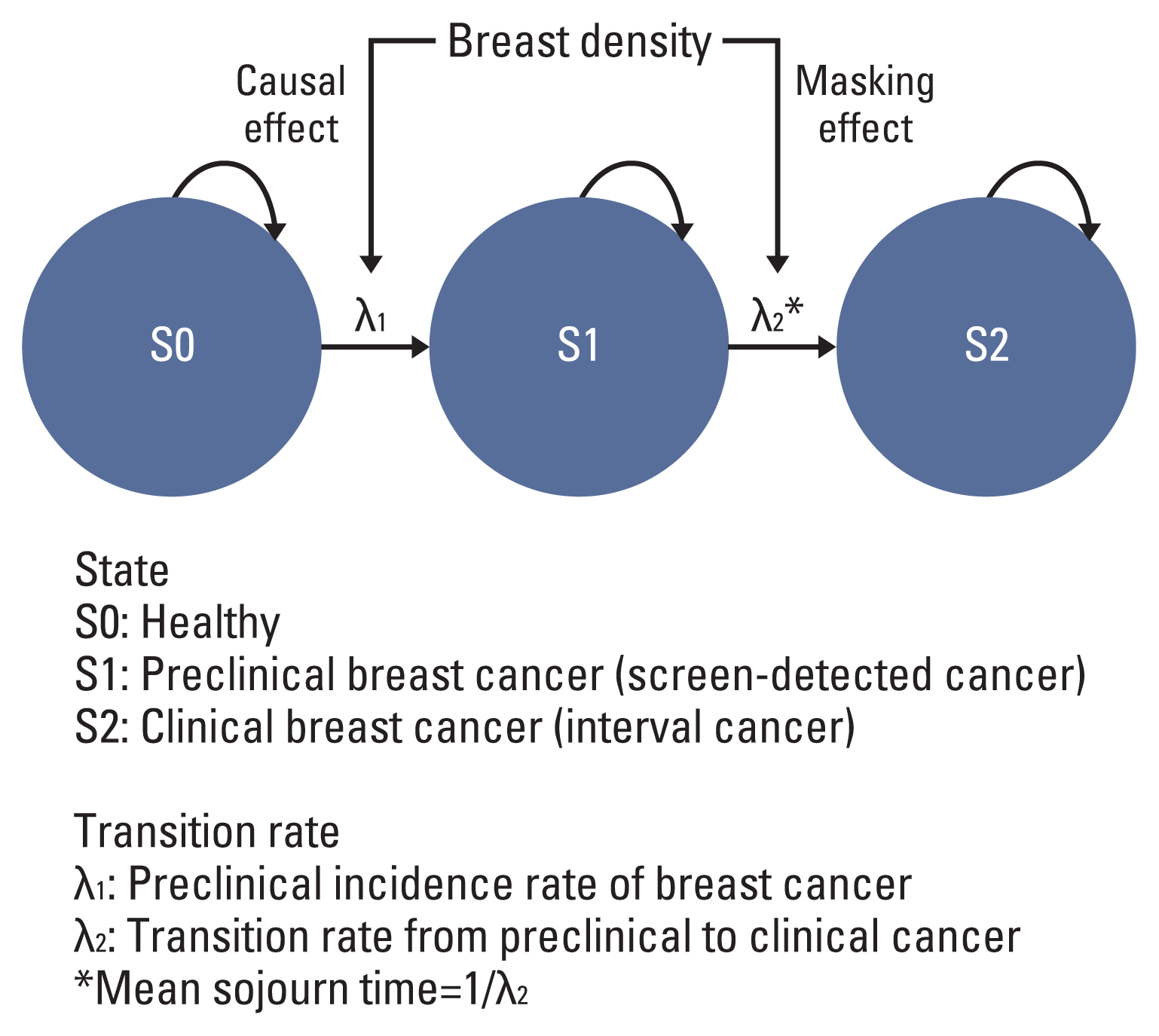
Fig. 2
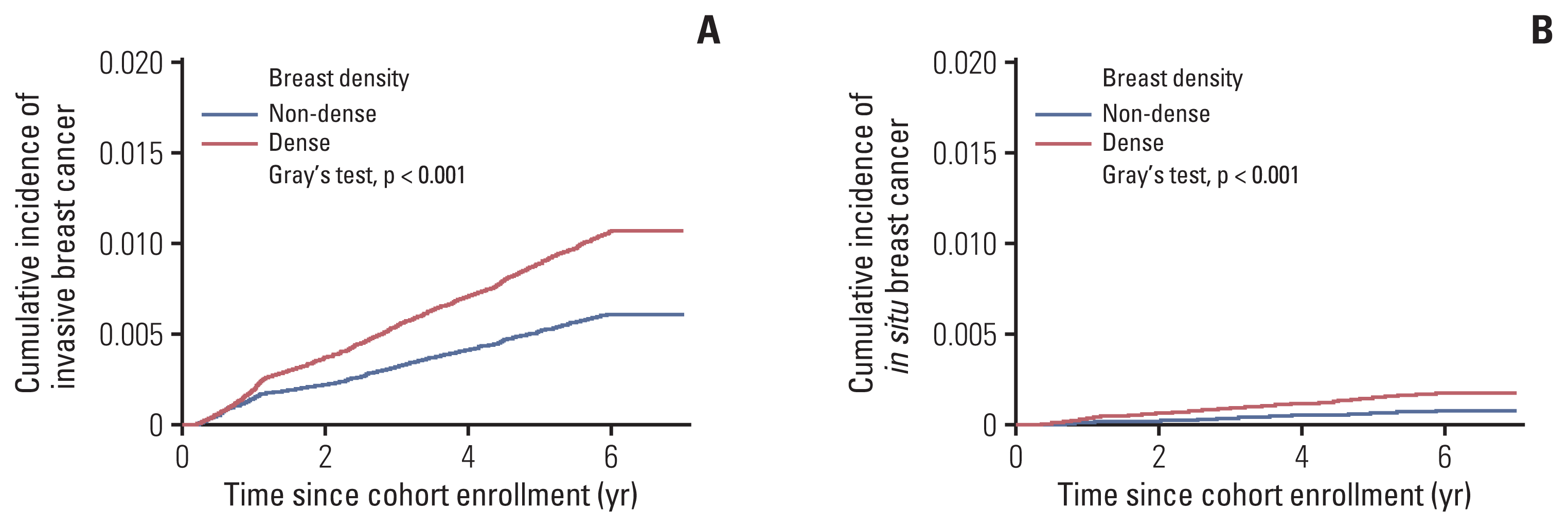
Table 1
| Age group (yr) | Total | % of density | Dense breast | Non-dense breast | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| No. screened | Interval cancer | Screen-detected cancer | I/S ratioa) | No. screened | Interval cancer | Screen-detected cancer | I/S ratioa) | No. screened | Interval cancer | Screen-detected cancer | I/S ratioa) | ||
| Total | 290,448 | 451 | 782 | 0.58 | 53.86 | 156,445 | 315 | 492 | 0.64 | 134,003 | 136 | 290 | 0.46 |
|
|
|||||||||||||
| 40–49 | 153,124 | 272 | 401 | 0.68 | 72.44 | 110,916 | 215 | 321 | 0.67 | 42,208 | 57 | 80 | 0.71 |
|
|
|||||||||||||
| 50–59 | 75,424 | 127 | 233 | 0.55 | 45.94 | 34,651 | 83 | 128 | 0.65 | 40,773 | 44 | 105 | 0.42 |
|
|
|||||||||||||
| 60–69 | 41,551 | 44 | 111 | 0.40 | 21.70 | 9,017 | 17 | 36 | 0.47 | 32,534 | 27 | 75 | 0.36 |
|
|
|||||||||||||
| ≥ 70 | 20,349 | 8 | 37 | 0.22 | 9.15 | 1,861 | 0 | 7 | 0.00 | 18,488 | 8 | 30 | 0.27 |
Table 2
Table 3
| Three-state Markov model for all breast cancers | Total | 40–49 | 50–59 | 60–69 |
|---|---|---|---|---|
| Preclinical incidence rate (λ1) | 0.0018 (0.0017–0.0019) | 0.0019 (0.0017–0.0021) | 0.0020 (0.0017–0.0022) | 0.0014 (0.0012–0.0017) |
| Mean sojourn time (1/λ 2 ) | 2.3940 (2.0918–2.7398) | 1.9772 (1.6707–2.3399) | 2.4936 (1.9252–3.2299) | 3.0717 (2.1126–4.4662) |
| Sensitivity of test | 0.67 (0.62–0.72) | 0.61 (0.54–0.61) | 0.65 (0.59–0.77) | 0.70 (0.62–0.77) |
| Hazard ratio by BI-RADS levels a) to transition rate | ||||
| Transition from healthy to preclinical (λ1) | ||||
| Level 2 vs. Level 1 | 1.9625 (1.5799–2.4370) | 1.3655 (0.9185–2.0300) | 1.9400 (1.3390–2.8110) | 1.9601 (1.5891–2.4170) |
| Level 3 vs. Level 1 | 2.3510 (1.9200–2.8780) | 1.7721 (1.2311–2.5500) | 2.6324 (1.8417–3.7620) | 2.2083 (1.7236–2.8290) |
| Level 4 vs. Level 1 | 2.3380 (1.8760–2.9140) | 1.9122 (1.3223–2.7640) | 2.3875 (1.5545–3.6650) | 3.5950 (2.4920–5.1850) |
| Transition from preclinical to clinical (λ2) | ||||
| Level 2 vs. Level 1 | 1.5285 (0.9915–2.3550) | 2.0632 (0.8106–5.2510) | 1.3981 (0.5712–3.4220) | 1.4073 (0.9266–2.1380) |
| Level 3 vs. Level 1 | 2.0230 (1.3340–3.0680) | 1.9771 (0.8162–4.7900) | 1.9442 (0.8094–4.6690) | 1.5950 (0.9891–2.5720) |
| Level 4 vs. Level 1 | 1.9360 (1.2240–3.0630) | 1.6214 (0.6538–4.0170) | 2.2383 (0.8626–5.8080) | 2.1330 (1.1020–4.1270) |
| Mean sojourn time by BI-RADS levels a) | ||||
| Predominantly fatty | 3.8923 (2.6077–5.8097) | 3.5241 (1.5046–8.2538) | 3.8394 (1.6505–8.9313) | 4.2303 (2.3965–7.4623) |
| Scattered fibroglandular | 2.5473 (2.0584–3.1524) | 1.7082 (1.2330–2.3666) | 2.7463 (1.9118–3.9451) | 2.8843 (2.0214–4.1155) |
| Heterogeneously dense | 1.9237 (1.6312–2.2686) | 1.7823 (1.4272–2.2257) | 1.9750 (1.4883–2.6208) | 1.9665 (1.2572–3.0760) |
| Extremely dense | 2.0100 (1.6187–2.4960) | 2.1745 (1.6745–2.8239) | 1.7153 (1.1002–2.6742) | 1.3408 (0.6412–2.8038) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download