Introduction
Materials and Methods
1. Study design and patient population
Fig. 1
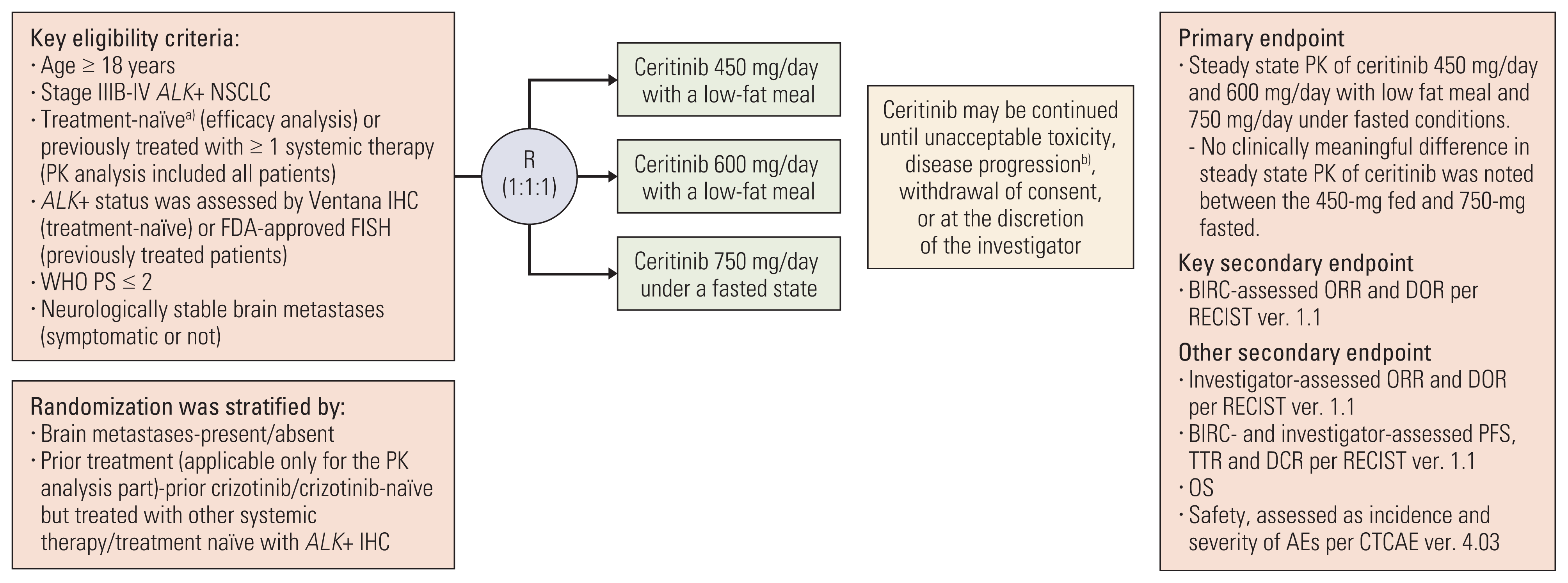
2. Endpoints
3. Assessments
4. Statistical analyses
Results
1. Patient characteristics and disposition
Table 1
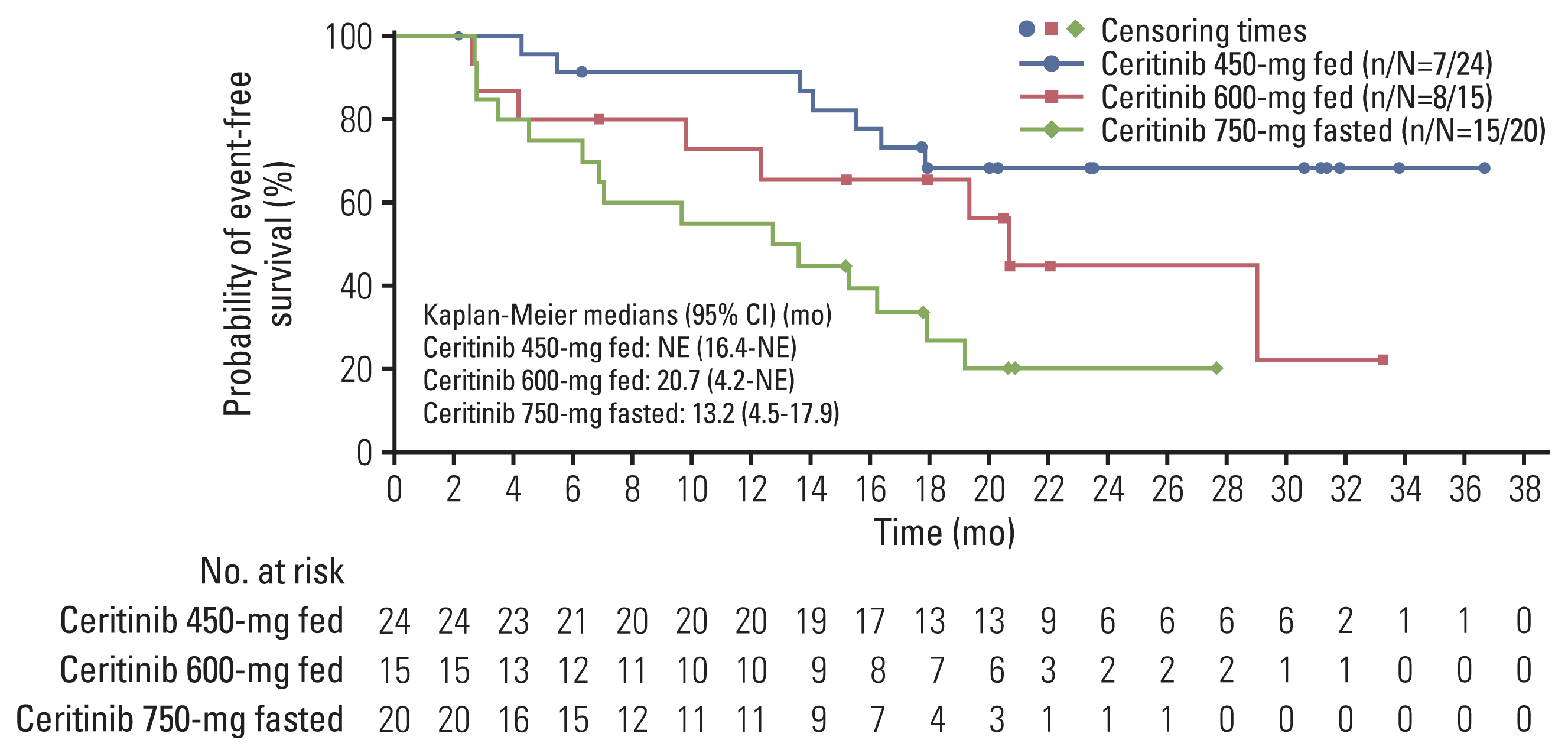
2. Efficacy
1) Response to ceritinib therapy
Fig. 2
Journal List > Cancer Res Treat > v.55(1) > 1516081056


Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Ethical Statement
The study protocol was approved by the independent ethics committee and/or institutional review board for each center as per the local regulations. This clinical study was designed and implemented in accordance with the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonization, with applicable local regulations. Written informed consent was obtained from each patient before screening.
Author Contributions
Conceived and designed the analysis: Cho BC, Geater S.
Collected the data: Cho BC, Kim DW, Batra U, Park K, Kim SW, Yang CT, Voon PJ, Sriuranpong V, Babu KG, Geater S.
Contributed data or analysis tools: Cho BC, Kim DW, Batra U, Park K, Kim SW, Yang CT, Voon PJ, Sriuranpong V, Babu KG, Amin K, Wang Y, Sen P, Slimane K, Geater S.
Performed the analysis: Cho BC, Kim DW, Batra U, Park K, Kim SW, Yang CT, Voon PJ, Sriuranpong V, Babu KG, Amin K, Wang Y, Sen P, Slimane K, Geater S.
Wrote the paper: Cho BC, Kim DW, Batra U, Park K, Kim SW, Yang CT, Voon PJ, Sriuranpong V, Babu KG, Amin K, Wang Y, Sen P, Slimane K, Geater S.
Conflicts of Interest
Byoung Chul Cho has received research funding from Novartis, Bayer, AstraZeneca, MOGAM Institute, Dong-A ST, Champions Oncology, Janssen, Yuhan, Ono, Dizal Pharma, Merck, Sharp & Dohme, Abbvie, Medpacto, GIInnovation, Eli Lilly, Blueprint medicines and Interpark Bio Convergence Corp. He also receives royalty from Champions Oncology. He is a consultant for Novartis, AstraZeneca, Boehringer-Ingelheim, Roche, Bristol Myers Squibb, Ono, Yuhan, Pfizer, Eli Lilly, Janssen, Takeda, Merck, Sharp & Dohme, Janssen, Medpacto, Blueprint medicines and serves on the advisory board of KANAPH Therapeutic Inc, Brigebio therapeutics, Cyrus therapeutics, Guardant Health, Joseah BIO. He is on the board of directors for Gencurix Inc and Interpark Bio Convergence Corp. He is a founder of the DAAN Biotherapeutics and owns stocks of TheraCanVac Inc, Gencurix Inc, Bridgebio therapeutics, KANAPH Therapeutic Inc, Cyrus therapeutics and Interpark Bio Convergence Corp.
Dong-Wan Kim declares research funding to his institution from Novartis, Alpha Biopharma, Amgen, Astrazeneca/Medimmune, Boehringer-Ingelheim, Daiichi-Sankyo, Hanmi, Janssen, Merus, Mirati Therapeutics, Merck, Sharp & Dohme, Ono Pharmaceutical, Pfizer, Roche/Genentech, Takeda, TP Therapeutics, Xcovery, Yuhan, Chong Keun Dang, Bridge BioTherapeutics and GlaxoSmithKline. He serves on the advisory board of Amgen, AsatraZeneca, Bristol Myers Squibb/Ono, Daiichi-Sankyo, GlaxoSmithKline, Merck, Sharp & Dohme, Janssen, Pfizer, SK Biopharm, Takeda, Yuhan and also reports travel support for advisory board meetings from Amgen and Daiichi-Sankyo.
Virote Sriuranpong has received research grants from AsraZeneca, Merck, Sharp & Dohme, Novartis, Regeneron, Roche and honoraria for lectures from AsraZeneca, Astellas, Bristol Myers Squibb, Novartis, Pfizer, and Roche.
Ullas Batra, Keunchil Park, Sang-We Kim, Cheng-Ta Yang, Pei-Jye Voon, K. Govind Babu, Sarayut Geater have nothing to disclose.
Khalid Amin, Yingbo Wang, Paramita Sen and Khemaies Slimane are employees of Novartis and own stocks/shares.