Abstract
Endometriosis is a relatively common gynecological condition in women of reproductive age. The rectosigmoid region is the most commonly affected segment when the gastrointestinal tract is involved. A differential diagnosis of colorectal neoplasia is difficult because of the similar clinical, endoscopic, and radiology findings. A 42-year-old female presented with abdominal distention and was subsequently diagnosed with a large bowel obstruction in the rectum. A temporary colostomy was performed, and endoscopy revealed a rectal mass obstructing the rectum. The biopsy showed normal mucosa, and it was difficult to exclude rectal malignancies even after the imaging workup. Endoscopic ultrasound demonstrated a hypoechoic lesion below the rectal mucosa, and fine needle aspiration confirmed the diagnosis of bowel endometriosis. Bowel endometriosis is a challenging diagnosis. Endoscopic ultrasound- guided fine-needle aspiration is useful for acquiring adequate samples for histological confirmation and a definitive diagnosis of bowel endometriosis.
Endometriosis is a common gynecological condition characterized by the abnormal growth of endometrial cells outside of the uterus.1 The condition affects primarily young women during their reproductive years, with an estimated prevalence of 10-15% of premenopausal women.2 The infiltration of ectopic endometrial tissue in the muscularis layer of the intestinal wall and surrounding structures can occur in 5-12% of patients with endometriosis.3 Regarding bowel endometriosis, the most commonly affected sites are the sigmoid colon and rectum in up to 90% of cases.4
The clinical presentation of endometriosis varies, and the symptoms are unspecific. Patients often present with dysmenorrhoea, dyspareunia, and infertility.1 Bowel-related symptoms, such as crampy abdominal pain, palpable abdominal mass, constipation, diarrhea, tenesmus, dyschezia, bowel obstruction, or intestinal bleeding, are dependent on the disease location and involvement of the bowel wall. They tend to be cyclic and intermittent, accompanying the menstrual cycle.1,3,5 On the other hand, the spectrum of disease severity is wide, and some women remain asymptomatic.3 Bowel endometriosis can be diagnosed incorrectly as a malignant tumor, inflammatory bowel disease, diverticulitis, ileocolonic intussusception, appendicitis, ischemic colitis, or other intestinal diseases, owing to its non-specific presentation.6
Radiological evaluation of bowel endometriosis can include transvaginal ultrasonography and MRI. An endoscopic evaluation can be useful when endometriosis infiltrates the mucosal layer. Histological confirmation with the identification of endometrial glands and stroma is essential for a definitive diagnosis because the differential diagnosis between bowel endometriosis and other diseases with a similar presentation is difficult with imaging alone.7 Hence, diagnosing and managing bowel endometriosis is challenging in some cases. This paper reports a case of bowel endometriosis with large bowel obstruction diagnosed by endoscopic ultrasound-guided fine needle aspiration.
This paper reports a case of bowel endometriosis with large bowel obstruction diagnosed by endoscopic ultrasound-guided fine needle aspiration. The patient consent to publication and data sharing.
A 42-year-old female presented to the emergency department reporting colicky abdominal pain, abdominal distension, the cessation of bowel movements, and flatus emission. She denied intestinal bleeding, night sweats, or weight loss, and the symptoms had no clear relationship with her menstrual cycle. She had no relevant prior medical history or family history of colorectal malignancies. The physical examination revealed a severely distended and tympanitic abdomen without audible bowel sounds.
Abdominal and pelvic CT revealed small and large bowel distension and a stenotic lesion in the upper rectum without a clear margin from the cervix (Fig. 1). She was diagnosed with an acute complete large bowel obstruction. Considering the unclear etiology of the rectal mass and the need to relieve the severe intestinal dilation, a temporary colostomy was performed to prevent vascular compromise and perforation.
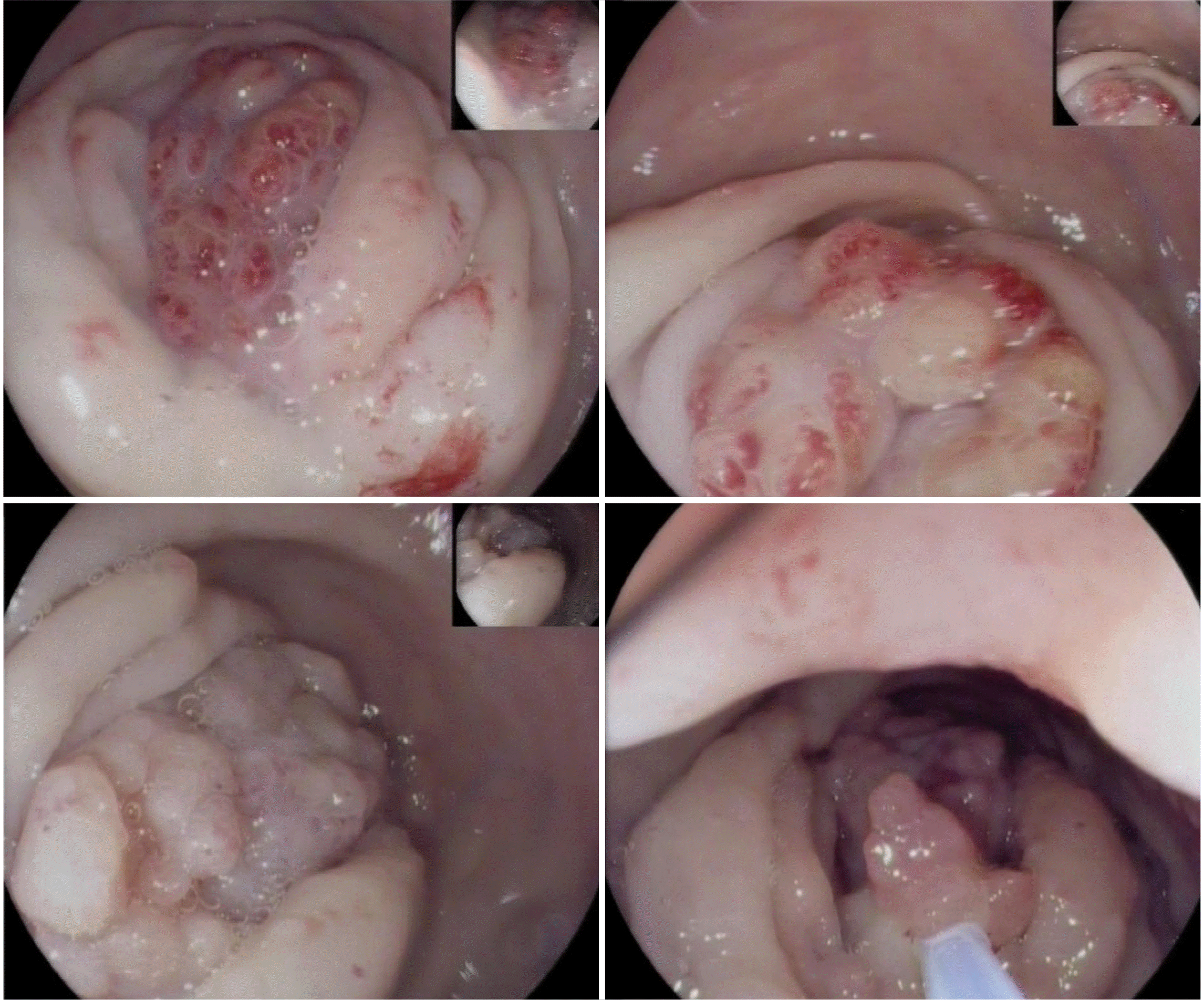
She underwent a rectosigmoidoscopy, which unveiled a pseudopolypoid stenotic lesion 10 cm above the anal verge, exhibiting surface reddening and granular changes suggestive of a submucosal glandular pattern, which caused complete obstruction of the upper rectum (Fig. 2). The endoscopist noted on the report that the lesion did not present with typical neoplastic features and suggested an alternative diagnosis of intestinal endometriosis. The anatomopathological evaluation of the transendoscopic biopsies confirmed a normal colonic mucosa without signs of malignancy.
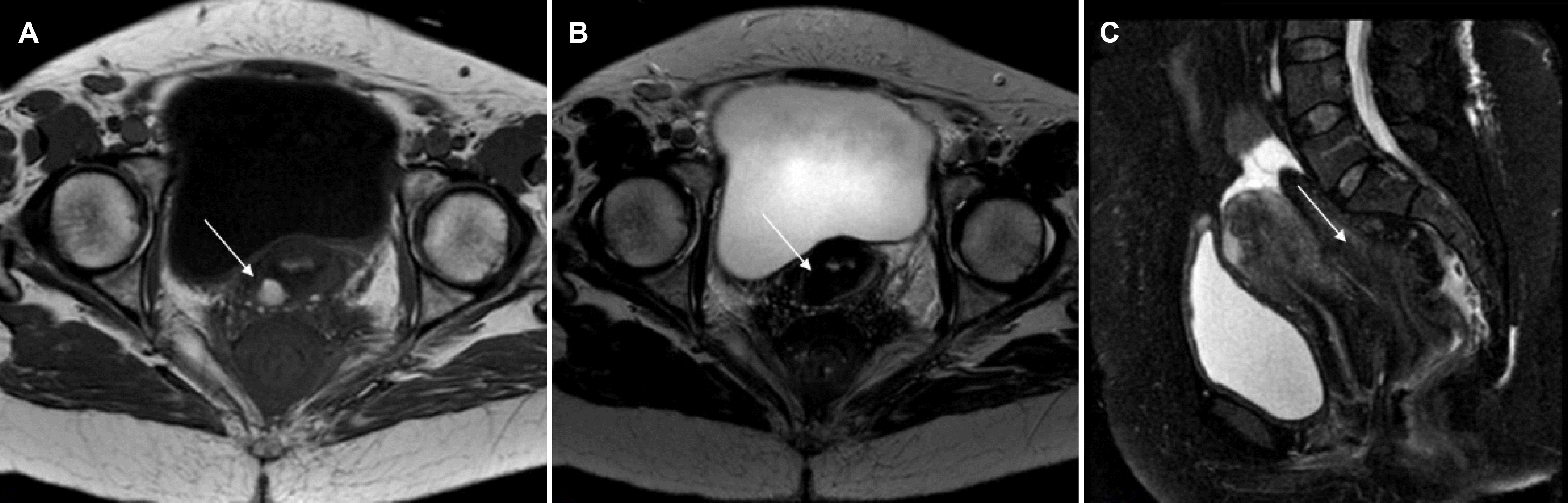
Imaging workup with pelvic MRI detected a lesion in the upper rectum (Fig. 3) with irregular circumferential thickening of the rectal wall, measuring 8.5 cm in length, invading the mesorectal fat and peritoneal reflection, without a clear margin between the lesion and the posterior cervical wall and levator ani muscles. There were also four lymphnodes in the mesorectal space, 10 mm in diameter, suggesting invasive rectal neoplasia. Cervical cytology was negative and the blood tests revealed a high level of serum cancer antigen 125 (106 U/mL; normal range 0-35 U/mL), but CEA and CA19.9 were within the normal range.
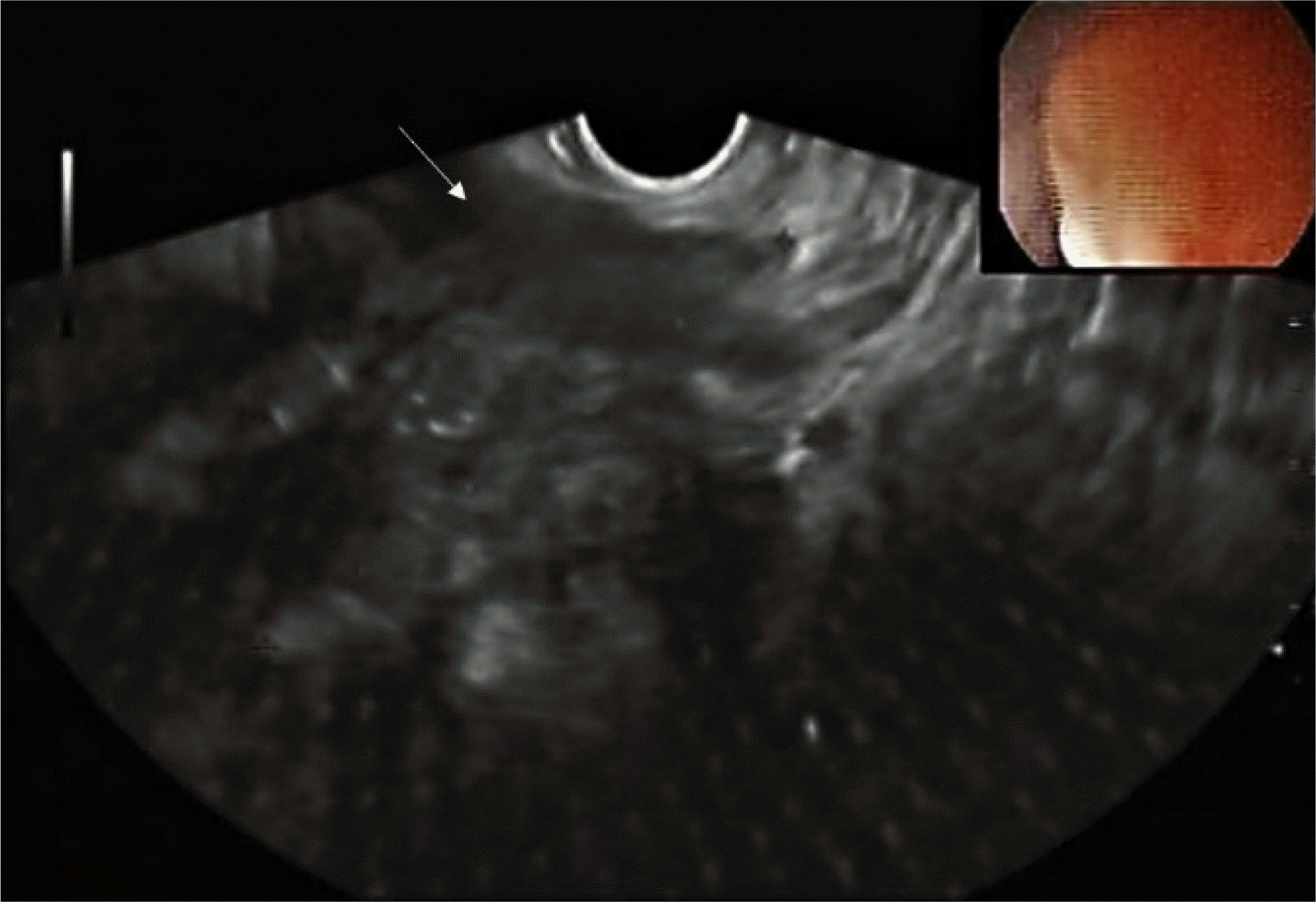
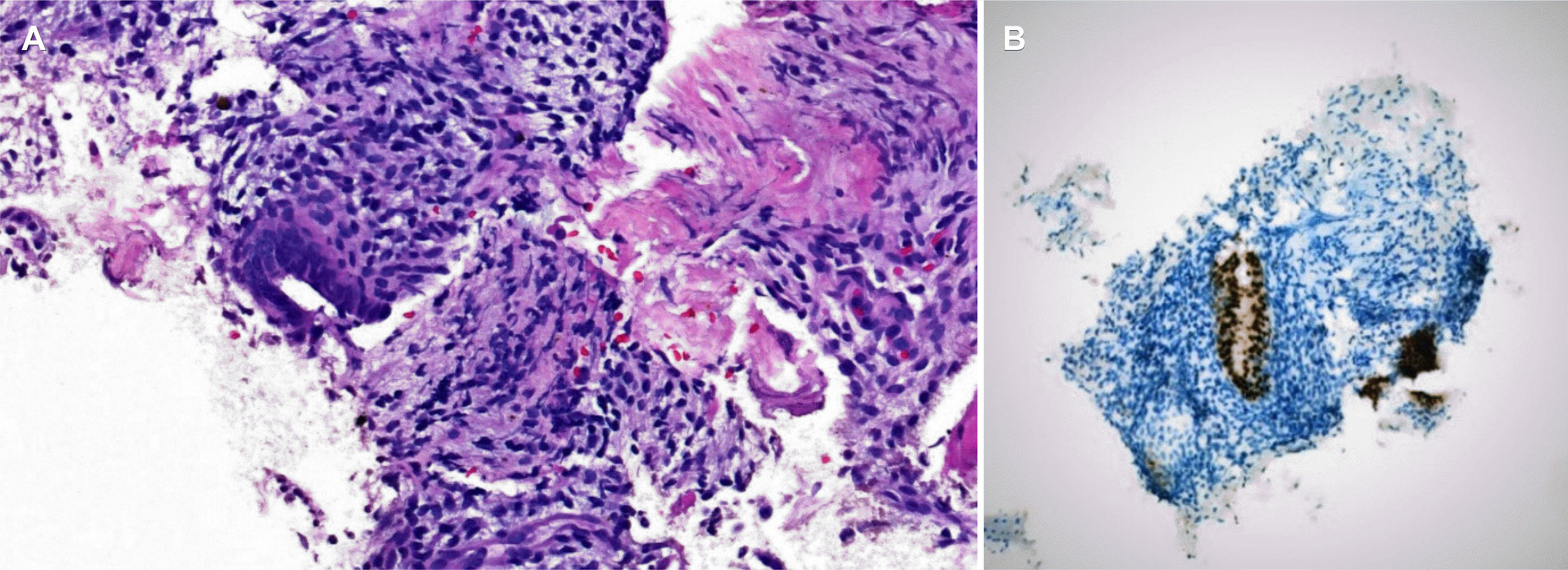
Subsequently, a rectal EUS was performed due to an inconclusive diagnosis and disagreement among the endoscopic, histologic, and radiology findings. The results demonstrated an irregular, hypoechoic, and heterogeneous mass below the rectal mucosa and submucosa, infiltrating the muscularis propria of the rectum (Fig. 4). After evaluation with a radial echoendoscope, a linear probe was used to perform fine-needle aspiration (FNA) of the lesion using a 22-gauge needle. The material was collected for the cell block procedure, and the pathology evaluation (Fig. 5) revealed ectopically located endometrial tissue dispersed among smooth muscle tissue. Immunohistochemical staining revealed a reactive epithelium to cytokeratin 7 and PAX8. Staining for CD117, SMA, S100, CK20, CD34, CDX2, and beta-catenin was negative. The final diagnosis was bowel endometriosis and there were no signs of malignancy.
The patient started hormonal therapy with triptorelin, but after six months, she developed additional pelvic and extrapelvic endometriotic lesions. She was then submitted for a total hysterectomy with bilateral oophorectomy and a proctosigmoidectomy with an end colostomy, with clinical improvement.
Bowel endometriosis often presents as a subepithelial lesion or an obstructive mass in the rectosigmoid area because of the involvement of the muscularis propria and subserosa.3 Superficial endometriotic implants confined to the serosa are often asymptomatic. On the other hand, if the muscularis layer is affected, it may lead to localized fibrosis, provoking severe gastrointestinal symptoms.6
The diagnostic workup is extensive and may require an interdisciplinary evaluation.8 In this patient with an acute large bowel obstruction, given the non-specific acute symptoms with no relation to the menstrual cycle, it was important to exclude a colonic malignancy because it requires different management and carries a different prognosis.
Radiologic and endoscopic workup is essential for a diagnosis, but there are relevant limitations. Transvaginal ultrasound allows an evaluation of the endometrium and uterus but does not exclude bowel endometriosis.1 Pelvic MR imaging performed with T1 (T1WI) and T2-weighting (T2WI) sequences are more specific than ultrasound or CT for this diagnosis of endometriosis. Fat-suppressed T1WI is also an important component, especially for assessing endometriosis.9 An endometrioma typically appears as a high-signal-intensity lesion on T1WI and fat-suppressed T1WI with low signal intensity in T2WI (T2 shading) because of repeated bleeding and blood degradation products.6,9 On the other hand, MRI has low sensitivity for detecting rectal lesions and low resolution to assess the depth of bowel infiltration.6 Colonoscopy is often performed to exclude other intestinal diseases with similar symptoms, but few endoscopists report findings suggestive of intestinal endometriosis, such as erythema, polypoid lesion or large masses, eccentric wall thickening, and surface nodularity and granularity.10
Thus, it is difficult to make a definitive diagnosis preoperatively because there are no disease-specific radiological or endoscopic findings for lesions with intact intestinal mucosa when biopsies generally yield insufficient tissue for a correct histological diagnosis.8 Among the imaging options, EUS has been used for more than 20 years to evaluate bowel endometriosis in a reliable and minimally invasive way.11 Endometriosis lesions usually involve the intestinal wall in an inward manner, starting from the serosa and extending to involve the muscularis propria and submucosa, even though it rarely involves the mucosa.3 The EUS examination showed that bowel endometriotic implants typically appear as hypoechoic and heterogeneous lesions with irregular and unclear margins, involving the serosa and the muscularis propria layers of the rectal wall in a longitudinal, fusiform and sometimes spiculated pattern.3,12 EUS has been associated with higher accuracy and negative predictive value compared to pelvic MR imaging for the diagnosis of bowel endometriosis.13 Recently, a retrospective cohort showed that EUS has high positive predictive value and high negative predictive value as well as excellent diagnostic accuracy for rectosigmoid endometriosis.14
While elective laparoscopy is the gold standard for bowel endometriosis diagnosis, adding FNA to EUS might be a less invasive and reliable option to acquire pathological specimens of infiltrating lesions of the rectum and distal sigmoid colon, allowing histological identification of endometrial glands and stroma.15 Using FNA under EUS guidance to perform sampling of these lesions can increase the diagnostic yield when there is a high clinical suspicion but with endoscopic biopsies unable to provide a definitive diagnosis of endometriosis.8 Despite low complications, the efficacy of this technique in the preoperative diagnosis of rectosigmoid endometriosis has only been described in a few studies.15-18 Rectal EUS-FNA can avoid unnecessary surgical diagnostic explorations but has some drawbacks, such as a suboptimal diagnostic yield of FNA, mainly relatable to the number of needle passes and cellularity of the specimen, the difficulty, and risk of perforation associated with the deep insertion of the echoendoscope into the sigmoid colon or the hypothetical risk of seeding endometriotic cells into the peritoneal cavity.15,16,19
In the present case, a rectal malignancy was initially suspected based on the acute onset of the symptoms and imaging evaluation. On the other hand, the rectal lesion was easily accessible for linear EUS, and ultrasonography findings along with FNA proved otherwise, allowing a preoperative diagnosis of bowel endometriosis.13
Involving a multidisciplinary team is crucial for determining the best therapeutic options to address clinical and surgical factors, patient preferences, and impact on the quality of life.5,20 The main objectives of therapy are a symptomatic improvement, debulking disease load, and suppression and delay of its recurrence and progression.1 This can be tackled through medical management (hormonal suppression, NSAIDs, danazol, or aromatase inhibitors) or surgery depending on clinical severity and complications, such as bowel obstruction or perforation. In this regard, a preoperative diagnosis using EUS may help determine the choice between laparoscopy and laparotomy when a complete resection is indicated.18
In summary, this paper reported an atypical presentation of bowel endometriosis in which EUS-guided FNA was the key to diagnosis, obviating the need for a diagnostic surgical exploration. In women of reproductive age presenting with an intestinal lesion of uncertain etiology and non-specific radiologic and endoscopic findings, it is essential to consider bowel endometriosis as a differential diagnosis.
ACKNOWLEDGEMENTS
We thank IPATIMUP and Daniel Cardoso (M.D.) for providing histopathology and MRI images, respectively.
REFERENCES
1. Parasar P, Ozcan P, Terry KL. 2017; Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 6:34–41. DOI: 10.1007/s13669-017-0187-1. PMID: 29276652. PMCID: PMC5737931.

2. Giudice LC, Kao LC. 2004; Endometriosis. Lancet. 364:1789–1799. DOI: 10.1016/S0140-6736(04)17403-5. PMID: 15541453.

3. Habib N, Centini G, Lazzeri L, et al. 2020; Bowel endometriosis: current perspectives on diagnosis and treatment. Int J Womens Health. 12:35–47. DOI: 10.2147/IJWH.S190326. PMID: 32099483. PMCID: PMC6996110.
4. Roman H. FRIENDS group (French coloRectal Infiltrating ENDometriosis Study group). A national snapshot of the surgical management of deep infiltrating endometriosis of the rectum and colon in France in 2015: a multicenter series of 1135 cases. J Gynecol Obstet Hum Reprod. 2017; 46:159–165. DOI: 10.1016/j.jogoh.2016.09.004. PMID: 28403973.

5. Abrão MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. 2015; Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 21:329–339. DOI: 10.1093/humupd/dmv003. PMID: 25618908.

6. De Ceglie A, Bilardi C, Blanchi S, et al. 2008; Acute small bowel obstruction caused by endometriosis: a case report and review of the literature. World J Gastroenterol. 14:3430–3434. DOI: 10.3748/wjg.14.3430. PMID: 18528943. PMCID: PMC2716600.

7. Wolthuis AM, Meuleman C, Tomassetti C, D'Hooghe T, de Buck van Overstraeten A, D'Hoore A. 2014; Bowel endometriosis: colorectal surgeon's perspective in a multidisciplinary surgical team. World J Gastroenterol. 20:15616–15623. DOI: 10.3748/wjg.v20.i42.15616. PMID: 25400445. PMCID: PMC4229526.

8. Adam A, Narayanan M, Hachem C. 2018; Endoscopic appearance and management of recto-sigmoid endometriosis: case report. Gastroenterology Res. 11:326–328. DOI: 10.14740/gr1049w. PMID: 30116434. PMCID: PMC6089590.

9. Khashper A, Addley HC, Abourokbah N, Nougaret S, Sala E, Reinhold C. 2012; T2-hypointense adnexal lesions: an imaging algorithm. Radiographics. 32:1047–1064. DOI: 10.1148/rg.324115180. PMID: 22786993.

10. Tomiguchi J, Miyamoto H, Ozono K, et al. 2017; Preoperative diagnosis of intestinal endometriosis by magnifying colonoscopy and target biopsy. Case Rep Gastroenterol. 11:494–499. DOI: 10.1159/000475751. PMID: 29033768. PMCID: PMC5624264.

11. Schröder J, Löhnert M, Doniec JM, Dohrmann P. 1997; Endoluminal ultrasound diagnosis and operative management of rectal endometriosis. Dis Colon Rectum. 40:614–617. DOI: 10.1007/BF02055389. PMID: 9152194.

12. Shi B, Sun B, Zhao Q, Zhang X. 2020; EUS diagnosis of rectal endometriosis. VideoGIE. 6:105–107. DOI: 10.1016/j.vgie.2020.10.014. PMID: 33884342. PMCID: PMC7859544.

13. Alborzi S, Rasekhi A, Shomali Z, et al. 2018; Diagnostic accuracy of magnetic resonance imaging, transvaginal, and transrectal ultrasonography in deep infiltrating endometriosis. Medicine (Baltimore). 97:e9536. DOI: 10.1097/MD.0000000000009536. PMID: 29465552. PMCID: PMC5842011.

14. James TW, Fan YC, Schiff LD, Gangarosa LM. 2019; Lower endoscopic ultrasound in preoperative evaluation of rectosigmoid endometriosis. Endosc Int Open. 7:E837–E840. DOI: 10.1055/a-0901-7259. PMID: 31198849. PMCID: PMC6561764.

15. Miwa T, Iwashita T, Aiba M, et al. 2020; Endoscopic ultrasound-guided fine needle aspiration for the diagnosis of bowel endometriosis: a case report. Med Ultrason. 22:243–246. DOI: 10.11152/mu-2000. PMID: 32190855.

16. Pishvaian AC, Ahlawat SK, Garvin D, Haddad NG. 2006; Role of EUS and EUS-guided FNA in the diagnosis of symptomatic rectosigmoid endometriosis. Gastrointest Endosc. 63:331–335. DOI: 10.1016/j.gie.2005.06.019. PMID: 16427951.

17. Hara K, Yamao K, Ohashi K, et al. 2003; Endoscopic ultrasonography and endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of lower digestive tract disease. Endoscopy. 35:966–969. DOI: 10.1055/s-2003-43473. PMID: 14606022.

18. Roseau G, Dumontier I, Palazzo L, et al. 2000; Rectosigmoid endometriosis: endoscopic ultrasound features and clinical implications. Endoscopy. 32:525–530. DOI: 10.1055/s-2000-9008. PMID: 10917184.

19. Kishimoto K, Kawashima K, Moriyama I, et al. 2020; Sigmoid endometriosis diagnosed preoperatively using endoscopic ultrasound-guided fine-needle aspiration. Clin J Gastroenterol. 13:158–163. DOI: 10.1007/s12328-019-01046-x. PMID: 31549336.

20. Moawad GN, Klebanoff JS, Habib N, Bendifallah S. 2021; Colorectal endometriosis: ample data without definitive recommendations. Facts Views Vis Obgyn. 13:3–7. DOI: 10.52054/FVVO.13.1.006. PMID: 33889855. PMCID: PMC8051188.

Fig. 1
Pelvic CT transversal (A) and coronal (B) images revealing a stenotic lesion in the upper rectum (arrow), without a clear margin from the cervix, with upstream large and small bowel distension.
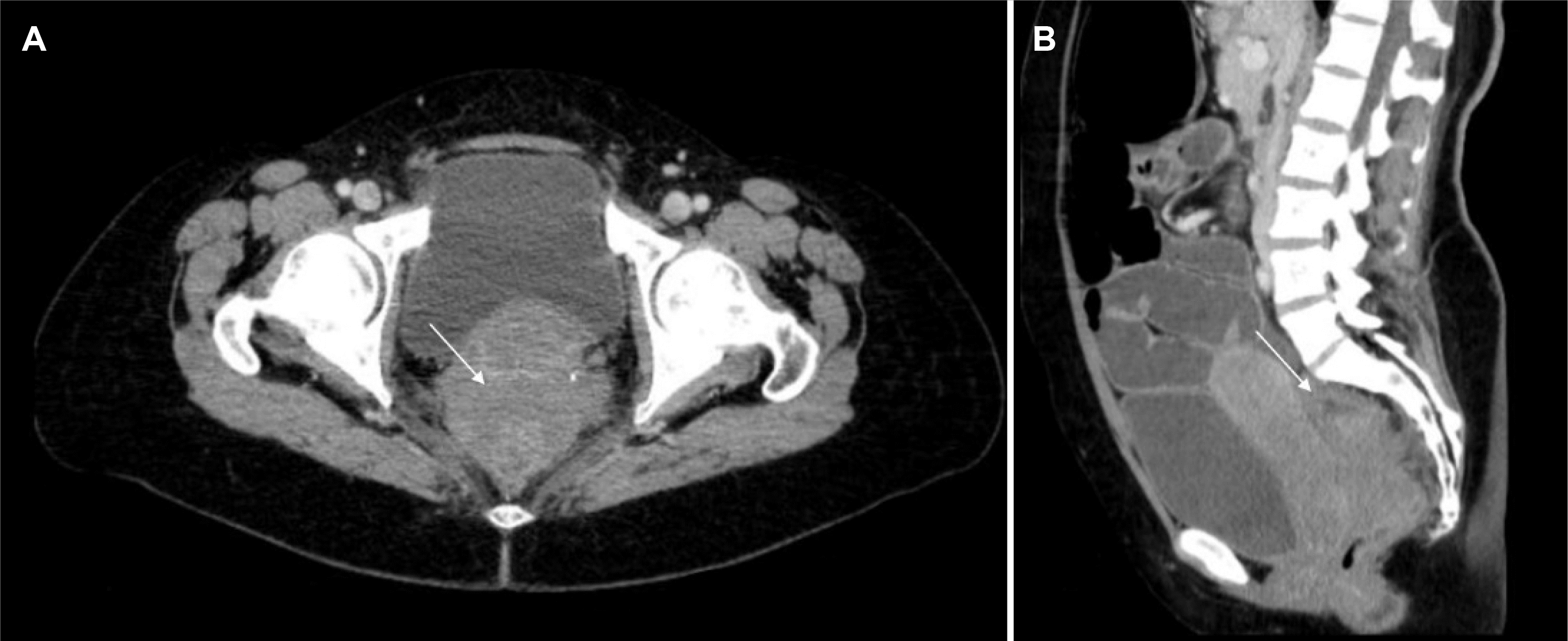
Fig. 3
Pelvic MRI T1 (A) and T2 weighted in transversal (B) and sagittal (C) view demonstrating a stenotic lesion (arrows) in the upper rectum and its anatomical relations with the uterus.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download