Abstract
An undifferentiated carcinoma (UC) of the gall bladder behaves aggressively and has a grave prognosis. Small cell type undifferentiated carcinoma of the gall bladder is a rare variant. This paper reports a case of UC of gall bladder with PAS-positive diastase- resistant eosinophilic hyaline globules present as liver mass (on imaging) in a male patient. The microscopic findings of the liver and gall bladder after a right tri-segmentectomy showed an un-differentiated malignant neoplasm composed of cells with round to oval nuclei, prominent nucleoli, and scanty neoplasm. No definite cell pattern was identified with these neoplastic cells. A section from the gall bladder revealed a tumor arising from the lining epithelium and infiltrating through the muscularis. This tumor was infiltrating the adherent liver tissue directly and forming a mass of undifferentiated malignant cells. The focal area within the tumor mass showed the presence of PAS-positive, diastase-resistant, eosinophilic hyaline globules within the neoplastic cells. The immunohistochemistry test was diffusely positive for perinuclear anti-neutrophil cytoplasmic antibodies and negative for chromogranin, vimentin, Desmin, alpha-fetoprotein, leukocyte common antigen, CD34, and bcl2. When the clinical and radiological data are inconclusive, careful analysis of the histological and immunophenotypic features is needed to make the final diagnosis of UC of the gall bladder. The biological behavior and prognosis of this tumor remain unclear because of its rarity. Further studies will be needed to understand the characteristics of this deadly tumor and to establish an effective therapy for it.
Small cell type undifferentiated carcinoma (UC) of the gallbladder are unusual neoplasms. Most of these tumors are diagnosed by radiological imaging as a mass in the gall bladder. Their presentation as liver masses and periodic acid-Schiff (PAS) positive, hyaline globules on a microscopic examination has never been reported. This paper reports the first such case of gall bladder carcinoma with a literature review of small cell variants of undifferentiated carcinoma.
The consent was taken from the legal guardian of the patient. A 41-year-old male was admitted to the authors’ hospital with a diagnosis of obstructive jaundice. The patient complained of myalgia, lethargy, weight loss, and upper abdominal pain for two months. He also had features of cholestasis-like, clay-colored stools and itching for two months. There was no history of fever, projectile vomiting, hematemesis, and black-colored stools. The patient had been diagnosed with type 2 diabetes mellitus (on oral hypoglycemic) and hemorrhoids.
The general examination revealed a yellow discoloration of the bulbar conjunctiva and skin. His vitals were stable. Mild tenderness was present over the right hypochondrium and epigastric region. There was no other remarkable finding on the general and systemic examinations.
The pertinent laboratory data on admission were suggestive of a deranged liver function test with raised total bilirubin (19.47 mg/dL; normal range: 0-1.2 mg/dL), high alkaline phosphatase (315 U/L; normal range: 40-129 U/L), mildly elevated serum glutamic oxaloacetic transaminase (167 U/L; normal range: 4-38 U/L) and serum glutamic pyruvic transaminase (130 U/L; normal range: 16-63). Tumor markers, including CEA (3.0 ng/mL; normal range: 0-5 ng/mL), alpha-fetoprotein (6.2 ng/mL; normal range: 0-7 ng/mL), and cancer antigen (CA) 19-9, were within the normal ranges. The serological test for hepatitis B and C were negative.
Radiological imaging was suggestive of an enlarged liver. Almost the entire right lobe of the liver and segment IVB showed a large, well-defined space-occupying lesion measuring 14.4 (AP)×13 (CC)×12 (TR) cm in size (Fig. 1A). This lesion showed inhomogeneous peripheral enhancement in the early and late arterial phases. Vessels were streaking centripetally into the area of central scarring with the progressive filling of contrast in the portal, hepatic and delayed phases. The mass was found compressing the liver hilum and causing minimal intrahepatic biliary radical dilatation. The gall bladder was normal in size and wall thickness (Fig. 1B) with no intraluminal filling defect/enhancing mass seen. No significant hepatoduodenal/mesenteric/pre-paraaortic or retroperitoneal lymphadenopathy was observed in the abdomen. The chest X-ray was normal.
Based on the clinical assessment, the laboratory result, and radiological imaging, a provisional diagnosis of a large right-side space-occupying lesion of the liver compressing the common hepatic duct, causing intrahepatic biliary radical dilatation was made. A liver biopsy of the mass lesion revealed the presence of sheets of large atypical cell infiltrates, but the immunohistochemistry (IHC) tests performed for glutamine synthetase, Glypican 3, HSP70, and other markers were inconclusive. The cells were positive for Pan cytokeratin (CK). Hence, the diagnosis of a poorly differentiated neoplasm was suggested.
In view of the adequate left liver remnant, a decision was made to perform a right tri-segmentectomy. Biliary decompression was performed prior, and preoperatively, bilirubin came to 6.5 mg/dL. The intraoperative findings were a large, highly vascular right-sided liver mass with a normal-appearing gall bladder. The call bladder was densely adherent to the liver. No extrahepatic disease was noted. No regional lymph node metastases were identified. The patient underwent a right trisectionectomy with a hepaticojejunostomy. The post-operative recovery was uneventful, with an improvement in the bilirubin level.
Serial slicing of the resected specimen showed a soft hemorrhagic mass, 12×10 cm in size, with a small satellite nodule of 2×2 cm in size. The gall bladder was adherent to the liver bed with focal thickening of the wall and seen in continuation with the tumor mass, but no mass was identified in the lumen (Fig. 2).
A microscopic examination of the tumor mass revealed an un-differentiated malignant neoplasm composed of cells with round to oval nuclei, prominent nucleoli, and scanty cytoplasm. No definite cell pattern was identified with these neoplastic cells (Fig. 3). A section of the gall bladder revealed the tumor arising from the lining epithelium and infiltrating through the muscularis. This tumor was observed directly infiltrating the adherent liver tissue and forming a mass of undifferentiated malignant cells (Fig. 4). The focal area within the tumor mass showed the presence of PAS-positive, diastase- resistant, eosinophilic, hyaline globules within the neoplastic cell (Fig. 3). IHC revealed the tumor cells to be diffusely PanCK positive negative for vimentin, CD34, chromogranin, leukocyte common antigen (LCA), AFP, BCL2 negative with weak positivity for CK-19 (Fig. 5). Hence, a final diagnosis of UC originating from the gall bladder, infiltrating the liver, and forming a liver mass was made.
Postoperatively, the patient was advised to undergo chemotherapy, but the patient refused to take chemotherapy and went to an alternative medicine practitioner. Follow-up abdominal imaging was performed when he revisited because of abdominal pain nine months later, which revealed multiple hepatic metastases. The patient’s condition deteriorated rapidly, and he succumbed to multiple liver and brain metastasis 12 months after surgery.
UC is a high-grade malignant neoplasm that lacks differentiation and is composed wholly or partially of undifferentiated cells that retain the feature indicative of an epithelial origin only on immunohistochemical or ultrastructural grounds. They represent a grade IV tumor in the American Joint Committee on Cancer (AJCC) grading system.1 These tumors are called anaplastic carcinomas. They are clinically aggressive and usually fatal.
UC has been reported in various organs, such as the sinonasal tract, thyroid, and lung but very rarely reported in the gall bladder, with only a few small case series and case reports.2-5 The reported incidence of undifferentiated carcinoma in the gall bladder varies from 1.6 to 10.9%.2 Most cases are seen in the age group of 88-94 years,3 with a male-to-female ratio of 1:25.3
UC presents clinically with right upper quadrant pain, anorexia, weight loss,2,4 and very rarely with hemobilia.5 The tumor markers, such as CEA and cancer antigen19-9 (CA19-9), are usually normal in undifferentiated carcinoma.2 On ultrasound, most cases are observed as heterogeneous intraluminal masses in the gall bladder with a medium size of 5 cm. On the other hand, no definite intraluminal mass was noted in the index patient. The tumor was noted mainly as a thickening of the gall bladder masquerading as a liver mass.
Grossly, most cases are seen as a large mass varying from 1.3 cm to 10 cm in size, presenting as a large intra-luminal polypoid mass within the gall bladder lumen. Few cases present as an infiltrative mass.3 The polypoidal mass has a rounded, smooth contour and is not papillary, as seen in papillary neoplasm.2
Microscopically, the World Health Organization (WHO) has classified four variants of undifferentiated carcinoma:6
1) Spindle and Giant cell type: this is the most common type of UC. They are composed of a variable proportion of spindle cells, polygonal cells, and giant cells.7,8
2) Osteo-clastic giant cell type: morphologically, they resemble a giant cell tumor of the bone. They are composed of multinucleated osteoclastic giant cells and mononuclear cells.
3) Small cell type: composed mainly of sheets of round cells with prominent nucleoli.
4) Nodular and Lobular type: they are composed of nodules or lobules of neoplastic cells.
Immunohistochemical staining is essential for definite categorization and includes the positive staining of tumor cells for epithelial markers, such as PanCK, EMA, and negative staining for LCA, vimentin, B-cell lymphoma 2 (bcl-2), S-100, and chromogranin. The origin of this tumor is uncertain, and there are two schools of thought. One group believes that UC arises from the dedifferentiation of a preexisting well-differentiated tumor, while another believes that they originate as an already aggressive undifferentiated cancer.
Treatment comprises surgical excision with or without chemotherapy. A palliative chemotherapeutic regimen for sarcomatoid carcinoma, including gemcitabine and cis-diamminedichloro- platinum, as reported by Doval et al.9, can be utilized. However, most authors have not found chemotherapy to be beneficial. The prognosis of these cases is poor, with approximately 81% mortality within a year.3 The cause of death includes tumor recurrence in the liver or peritoneum.
Small cell UC is still a rarer type of UC, with only nine cases reported in the English literature thus far (Table 1). Most of these cases were seen in females (6/9) in the 44-75-year age group. Grossly these cases are seen as an intraluminal protruding mass in the gall bladder.
This paper reports the first case of small cell UC presenting as a liver mass. There is only a single case report of spindle cell type UC presenting as a liver mass.10 Presentation of an undifferentiated carcinoma of the gall bladder as a liver mass is rare and should be kept as a differential diagnosis of liver mass on imaging. All surgeons must understand the natural history, biology, and imaging feature of this variant of gall bladder cancer for its early diagnosis, which is vital for the patient’s survival.
Microscopically, these cases show a predominance of a small cell population. PAS-positive diastase-resistant globules are seen in cases of eosinophilic inclusions in the cytoplasm. Usually, intracytoplasmic glucose is diastase labile. The presence of diastase-resistant globules is indicative of a cytoplasmic content other than glucose, such as Alpha 1 antitrypsin and mucin. The pathology of undifferentiated carcinoma was an independent prognostic factor for poor survival in gall bladder cancer, as reported by Park et al.2 On the other hand, no intra-cytoplasmic globules were described previously. In the present case, there was the presence of focally intracytoplasmic PAS-positive diastase-resistant hyaline globules. Therefore, a differential diagnosis of embryonal sarcoma was made, but the immunohistochemical stains for the same (vimentin and bcl-2) were negative.
The pathology of undifferentiated carcinoma was an independent prognostic factor for poor survival in gall bladder cancer, as reported by Park et al.2 All patients except one with the small cell variant of undifferentiated gall bladder carcinoma died within one year of diagnosis. No case with similar morphological findings has been described previously in the English literature. The rarity of the small cell variant of undifferentiated carcinoma gall bladder poses significant limitations to the interpretation of our report. Given the limited literature, definite surgery and chemotherapy guidelines still need to be defined.
An undifferentiated carcinoma of the gall bladder presenting as a liver mass with eosinophilic intracytoplasmic globules is an extremely rare neoplasm. Given the paucity of available clinical data, they represent a clinical challenge in daily practice. Careful analysis of the histological and immunological features is also required, in addition to the clinical and radiological criteria for its prognostic significance.
Early diagnosis and surgical intervention with a better understanding of the tumor biology may offer improved prognosis and survival in this rare cancer. The pathological examination needs to be able to differentiate them from embryonal sarcoma because of overlapping features. Therefore, histological and IHC are essential for distinguishing these neoplasms. Embryonal sarcomas are positive for vimentin and are usually seen in children 6-10 years of age. By contrast, UC occurs in adults and is PanCK positive.
REFERENCES
1. National Cancer Institute, tumour grade. [Internet]. Available from: https://www.cancer.gov/about-cancer/diagnosis-staging/prognosis/tumour-grade-fact-sheet. National Cancer Institute;cited 2014 Aug 18.
2. Park HJ, Jang KT, Choi DW, Heo JS, Choi SH. 2014; Clinicopathologic analysis of undifferentiated carcinoma of the gallbladder. J Hepatobiliary Pancreat Sci. 21:58–63. DOI: 10.1002/jhbp.3. PMID: 23798378.

3. Guo KJ, Yamaguchi K, Enjoji M. 1988; Undifferentiated carcinoma of the gallbladder. A clinicopathologic, histochemical, and immunohistochemical study of 21 patients with a poor prognosis. Cancer. 61:1872–1879. DOI: 10.1002/1097-0142(19880501)61:9<1872::AID-CNCR2820610925>3.0.CO;2-Q. PMID: 2451557.

4. Manouras A, Genetzakis M, Lagoudianakis EE, et al. 2009; Undifferentiated giant cell type carcinoma of the gallbladder with sarcomatoid dedifferentiation: a case report and review of the literature. J Med Case Rep. 3:6496. DOI: 10.1186/1752-1947-3-6496. PMID: 19830109. PMCID: PMC2726484.

5. Kubota H, Kageoka M, Iwasaki H, et al. 2000; A patient with undifferentiated carcinoma of gallbladder presenting with hemobilia. J Gastroenterol. 35:63–68. DOI: 10.1007/PL00009979. PMID: 10632545.

6. Bosman FT. World Health Organization, International Agency for Research on Cancer. 2010. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer.
7. Nishihara K, Tsuneyoshi M. 1993; Undifferentiated spindle cell carcinoma of the gallbladder: a clinicopathologic, immunohistochemical, and flow cytometric study of 11 cases. Hum Pathol. 24:1298–1305. DOI: 10.1016/0046-8177(93)90263-G. PMID: 8276377.

8. Kubota K, Kakuta Y, Kawamura S, et al. 2006; Undifferentiated spindle-cell carcinoma of the gallbladder: an immunohistochemical study. J Hepatobiliary Pancreat Surg. 13:468–471. DOI: 10.1007/s00534-006-1100-x. PMID: 17013725.

9. Doval DC, Azam S, Mehta A, et al. 2014; A report of sarcomatoid carcinoma of the gallbladder treated with palliative deocetaxel and gemcitabine chemotherapy. J Gastrointest Cancer. 45 Suppl 1:270–274. DOI: 10.1007/s12029-014-9654-3. PMID: 25326734.

10. Akatsu T, Ueda M, Shimazu M, et al. 2005; Primary undifferentiated spindle-cell carcinoma of the gallbladder presenting as a liver tumor. J Gastroenterol. 40:993–998. DOI: 10.1007/s00535-005-1684-y. PMID: 16261437.

Fig. 1
Contrast-enhanced CT scan. (A) Image showing a normal appearing gall bladder with a heterogeneous mass in segments 5 and 6 (arrow). (B) Image shows intensely enhancing huge vascular mass lesion occupying almost the entire right lobe of the liver.
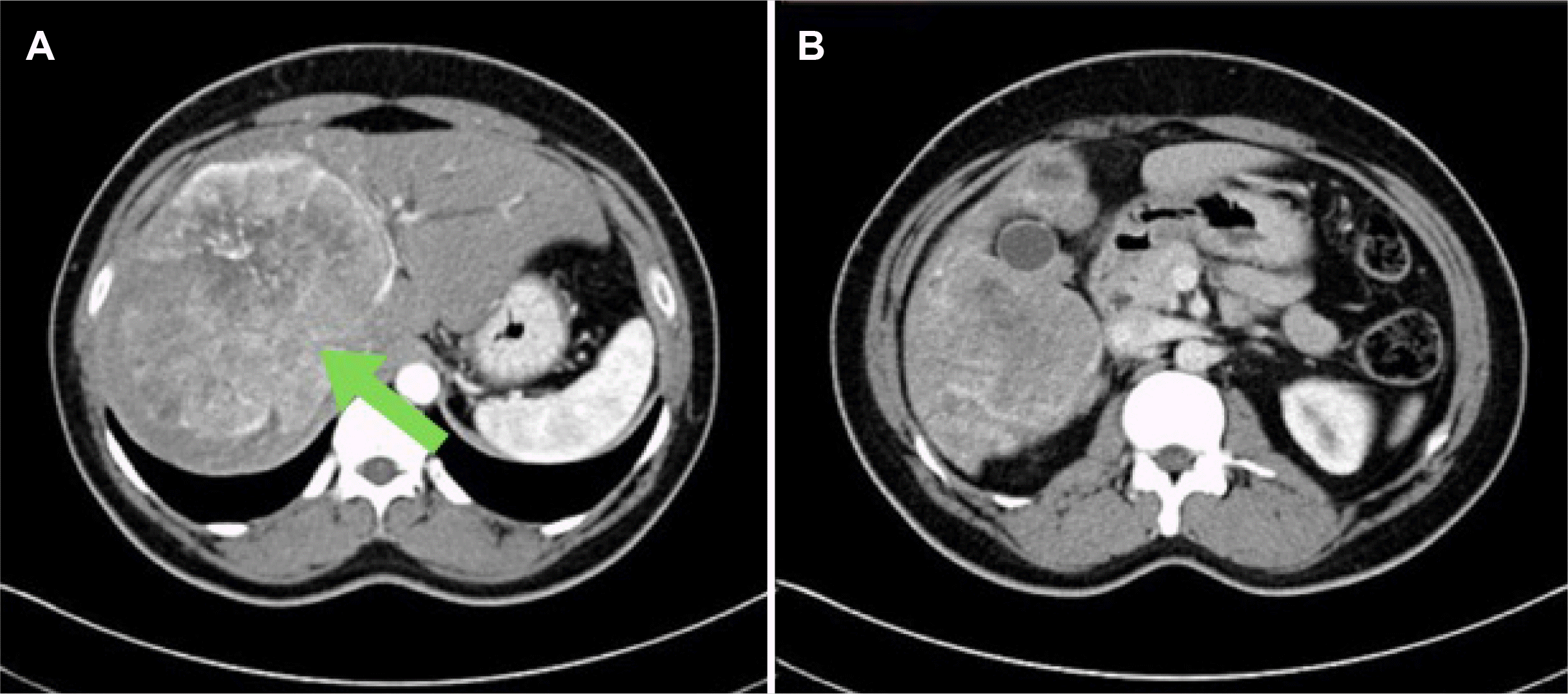
Fig. 2
Gross finding of the resected specimen of gall bladder carcinoma. (A) Gross showing a large liver mass. (B) Gross cut surface showing whitish large liver mass. (C) Gross of the liver with gall bladder showing an infiltrative mass from the gall bladder directly infiltrating the liver.
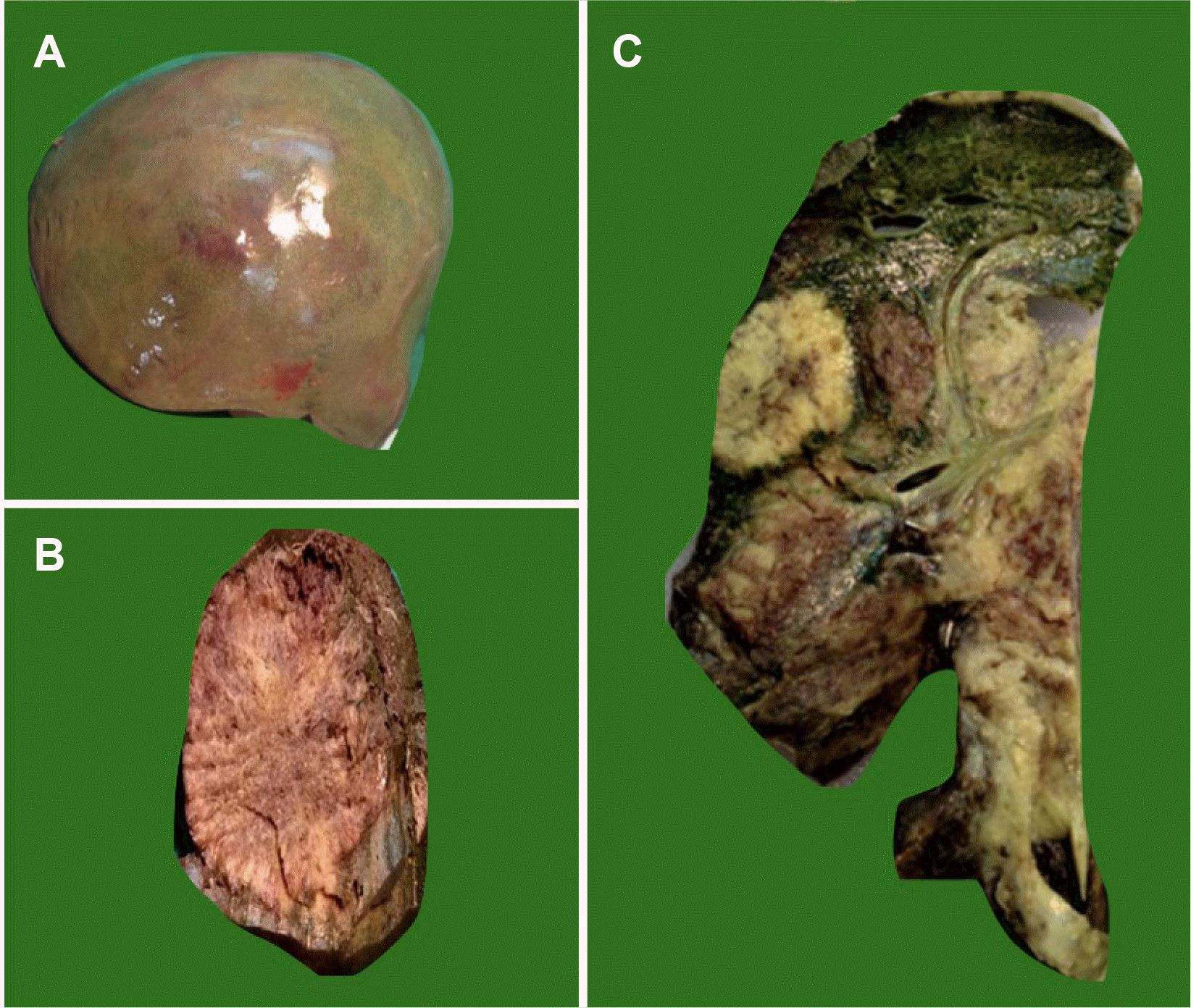
Fig. 3
Microscopic examination of the tumor mass. (A) Diffuse sheets of round tumor cells infiltrating hepatocytes (H&E, ×40). (B) Diffuse sheets of tumor cells (H&E, ×40). (C) Small round tumor cells (H&E, ×400). (D) PAS positive diastase resistant globules in the cytoplasm (arrow) (H&E, ×400). PAS, periodic acid-Schiff.
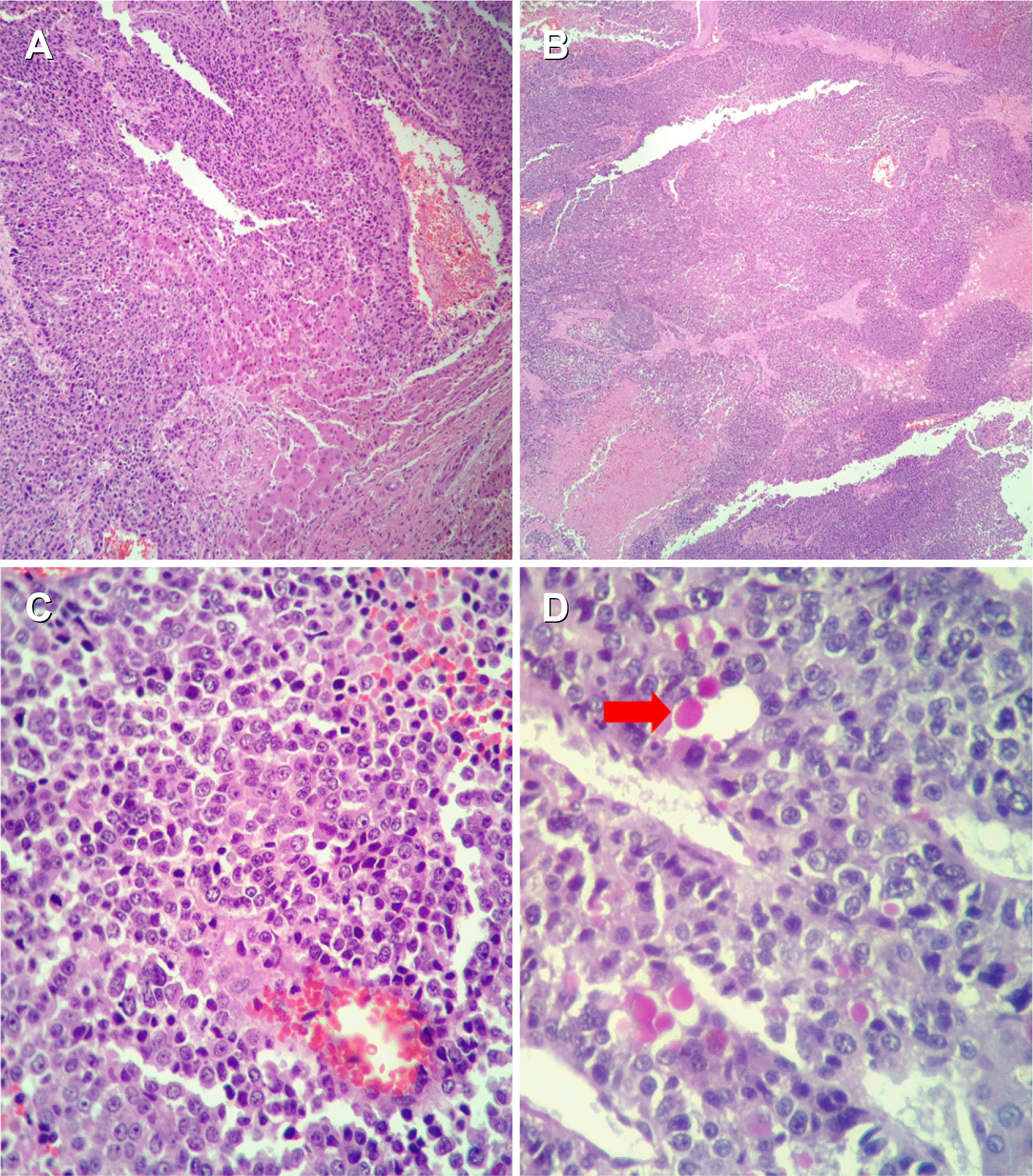
Fig. 4
Microscopic section from the gall bladder. (A) Tumor is seen arising from gall bladder (arrow) (H&E, ×40). (B) Malignant glands and cell nest (H&E, ×100). (C) Glands lined by malignant cells (H&E, ×400). (D) Tumor cells arising from lining epithelium (H&E, ×400).
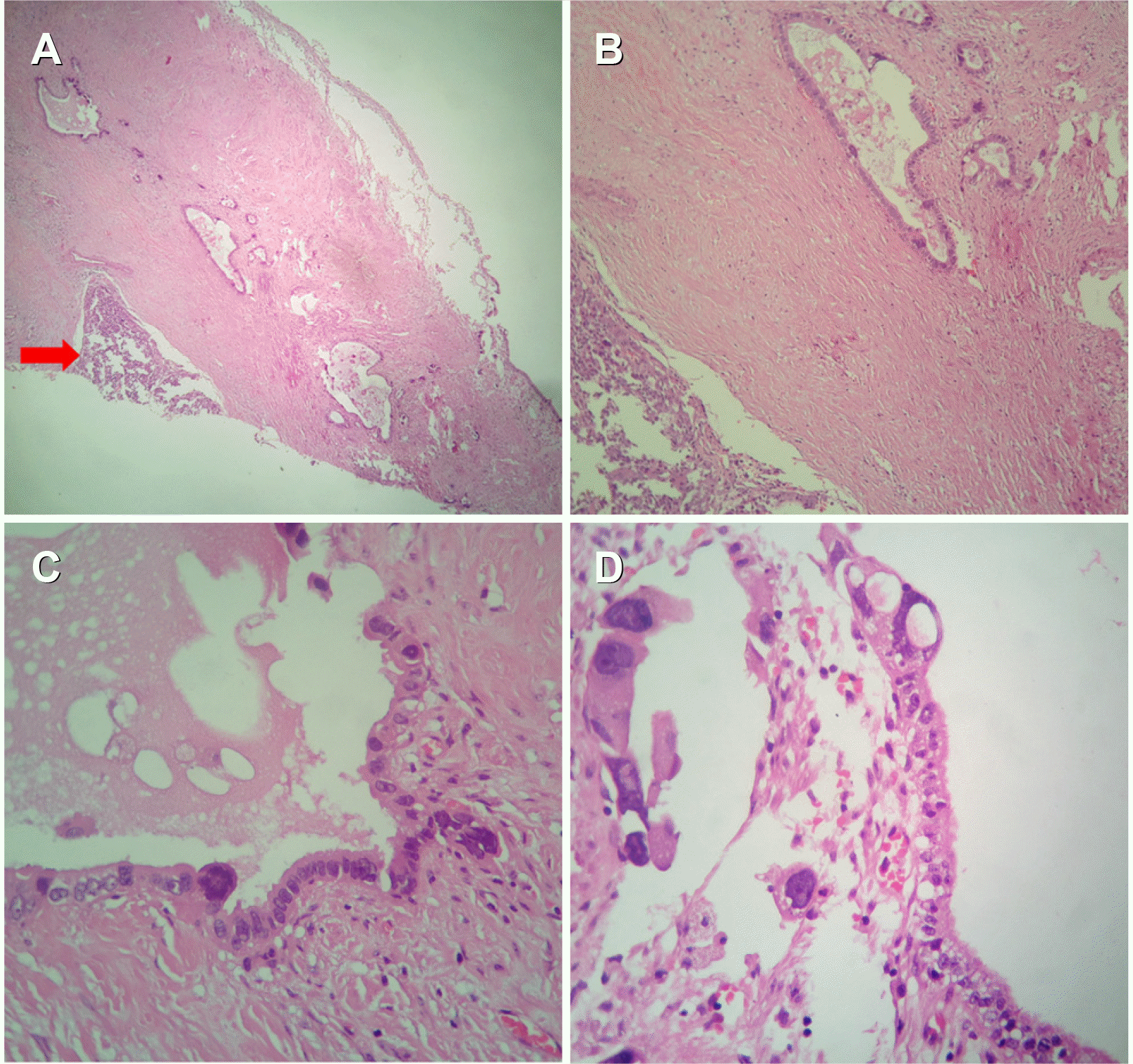
Fig. 5
Immuno-histochemistry. (A) IHC PanCK positive in tumor cells (H&E, ×100). (B) IHC LCA negative in tumor cells (H&E, ×100). (C) IHC vimentin negative in tumor cells (H&E, ×100). (D) IHC chromogranin negative in tumor cells (H&E, ×100). IHC, immunohistochemistry; LCA, leukocyte common antigen.
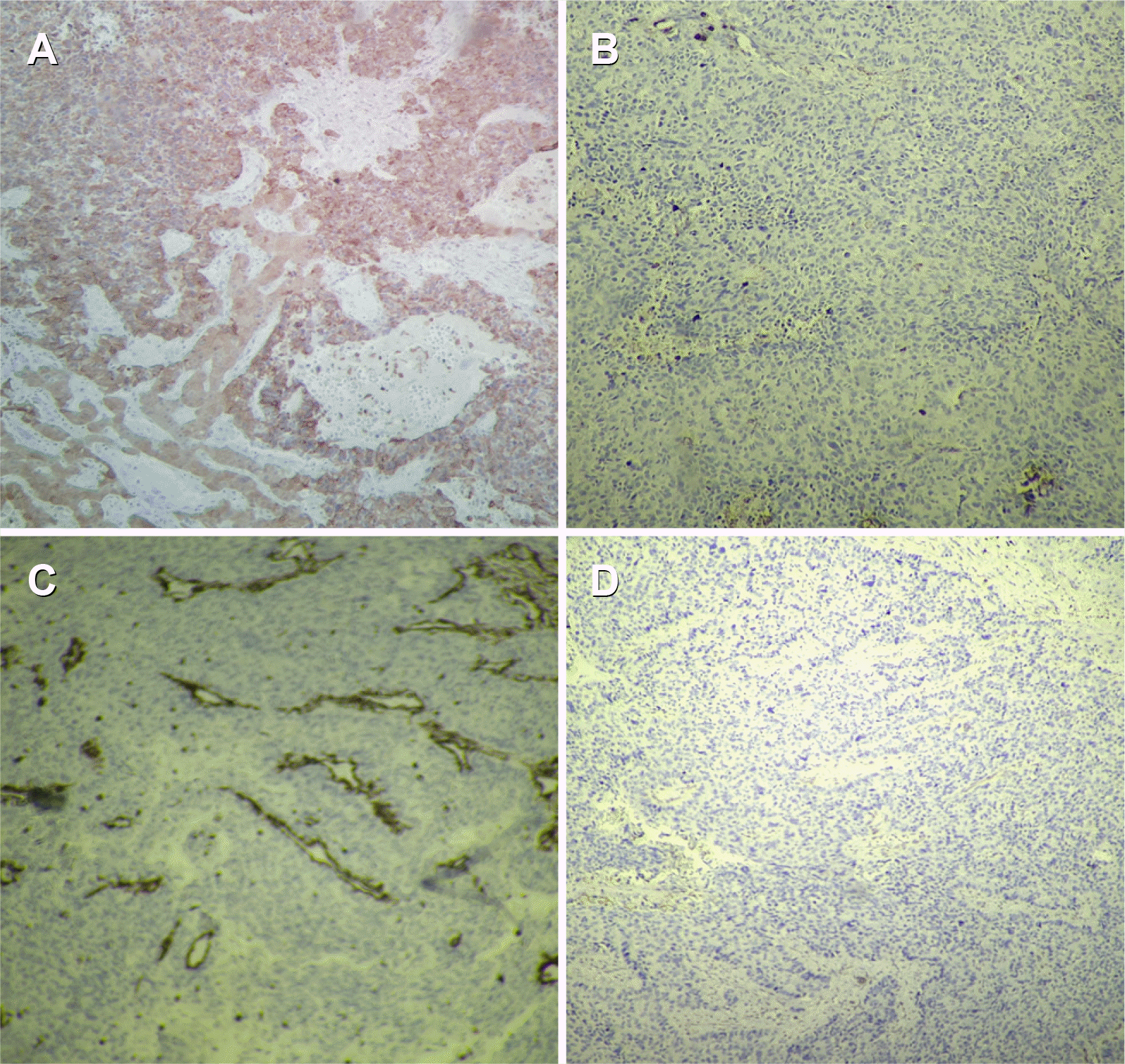
Table 1
Cases of Small Cell Undifferentiated Carcinoma of the Gall Bladder
| S.N. | Study | Journal | Cases | Age/Sex | Followup | Macroscopic |
|---|---|---|---|---|---|---|
| 1. | Guo et al.3 (1988) | Cancer | 1 | 44/M | Lost (follow up) | Protruded |
| 2 | 75/F | Died (9 month) | Unknown | |||
| 3 | 72/F | Died (2 month) | Protruded | |||
| 4 | 56/F | Died (2 month) | Protruded | |||
| 5 | 68/F | Live (59 month) | Protruded | |||
| 6 | 50/F | Autopsy | Protruded | |||
| 7 | 69/F | Autopsy | Infiltrating | |||
| 8 | 73/F | Autopsy | Protruded | |||
| 2 | Park et al.2 (2014) | Journal of hepatobiliary pancreas | 46/F | Died (7-8 month) | 5 cm tumour | |
| 3 | Our case (2020) | 41/M | Alive (at present) | Liver mass |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download