1. Raut CP, Kulke MH, Glickman JN, Swanson RS, Ashley SW. Carcinoid tumors. Curr Probl Surg. 2006; 43:383–450.
2. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008; 26:3063–72.
3. Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993; 20:716–31.
4. Krenning EP, de Jong M, Kooij PP, Breeman WA, Bakker WH, de Herder WW, et al. Radiolabelled somatostatin analogue(s) for peptide receptor scintigraphy and radionuclide therapy. Ann Oncol. 1999; 10(Suppl 2):S23–9.
5. Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011; 29:2416–23.
6. Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008; 26:2124–30.
7. Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with (1)(7)(7) Lu-DOTATATE: the IEO phase I–II study. Eur J Nucl Med Mol Imaging. 2011; 38:2125–35.
8. Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Horsch D, O’Dorisio MS, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013; 40:800–16.
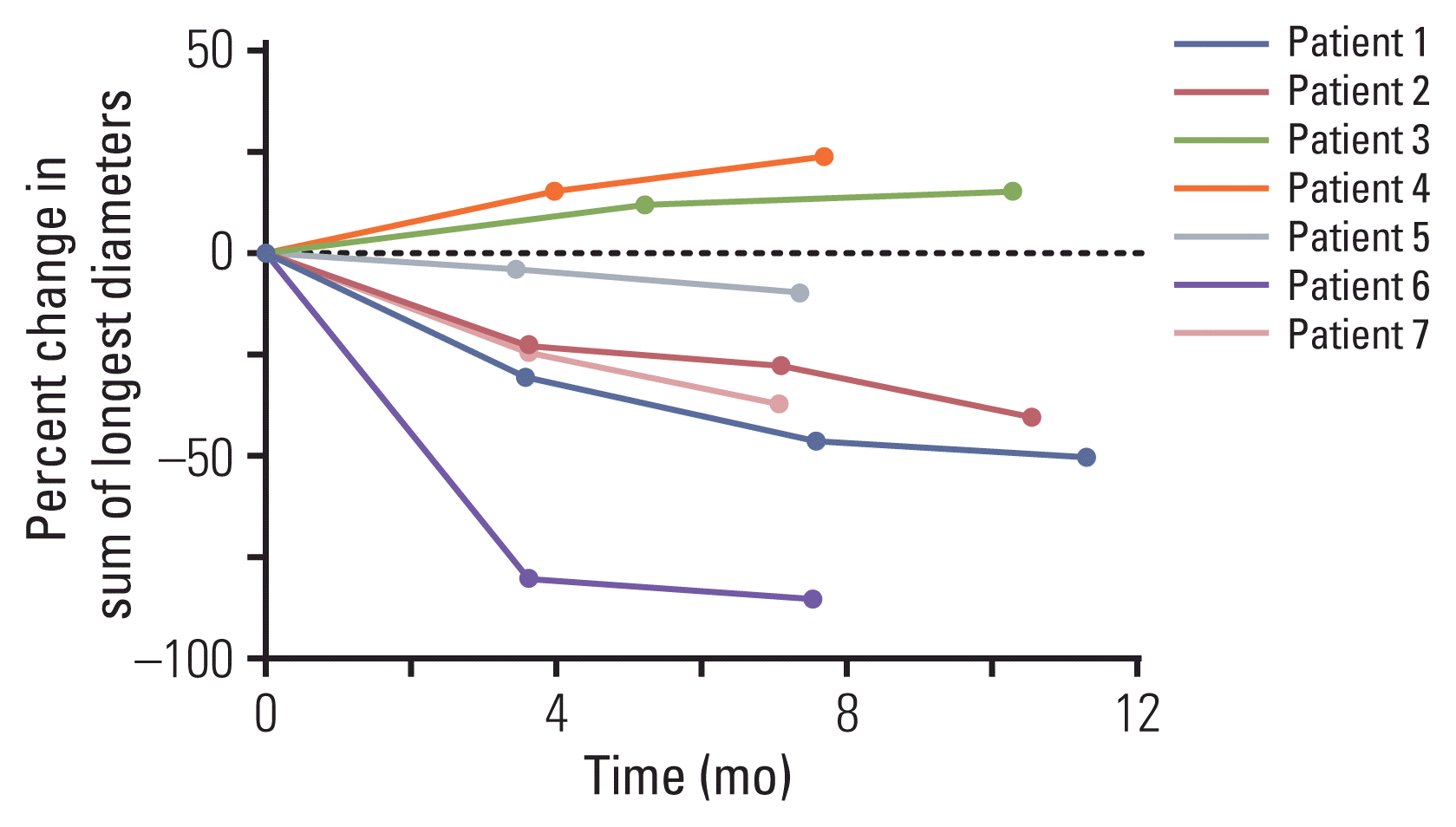
9. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of (177)Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017; 376:125–35.
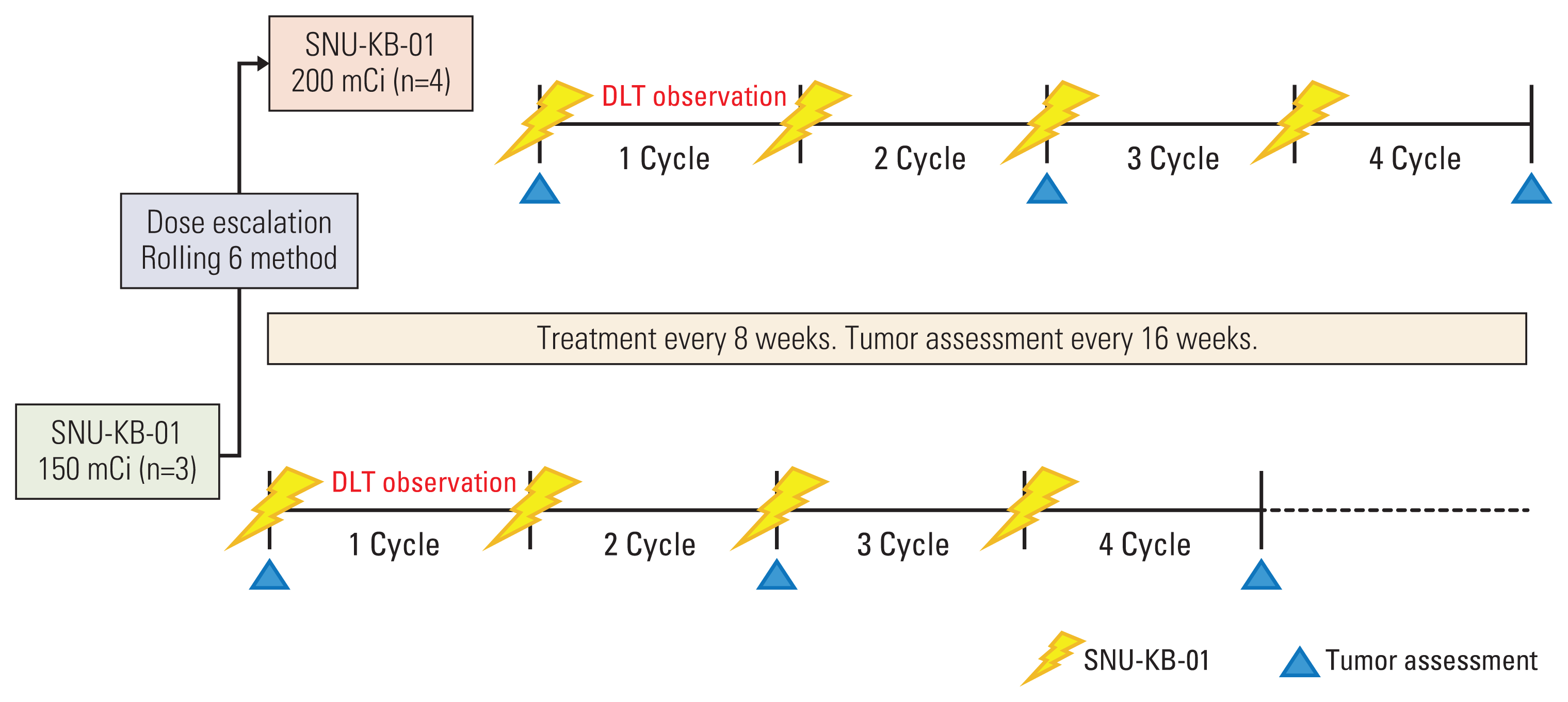
10. Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008; 26:190–5.
11. Coleman CN, Blakely WF, Fike JR, MacVittie TJ, Metting NF, Mitchell JB, et al. Molecular and cellular biology of moderate-dose (1–10 Gy) radiation and potential mechanisms of radiation protection: report of a workshop at Bethesda, Maryland, December 17–18, 2001. Radiat Res. 2003; 159:812–34.
12. Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991; 21:109–22.
13. Delpassand ES, Samarghandi A, Zamanian S, Wolin EM, Hamiditabar M, Espenan GD, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas. 2014; 43:518–25.
14. Dash A, Pillai MR, Knapp FF Jr. Production of (177)Lu for targeted radionuclide therapy: available options. Nucl Med Mol Imaging. 2015; 49:85–107.
15. Verberne HJ, Sokole EB, van Moerkerken AF, Deeterink JH, Ensing G, Stabin MG, et al. Clinical performance and radiation dosimetry of no-carrier-added vs carrier-added 123I-metaiodobenzylguanidine (MIBG) for the assessment of cardiac sympathetic nerve activity. Eur J Nucl Med Mol Imaging. 2008; 35:798–807.
16. Limouris G, Paphiti M, Karfis I, McCready V, Krylov V, Dolgushin M, et al. Progression free survival and response rate differences between non-carrier added (nca) and carrier added (ca) 177 Lu-DOTATATE. In: Non-functioning NET (NF-NET) liver metastasized, treated patient. Eur J Nucl Med Mol Imaging. 2018; 45(Suppl 1):S595.
17. Lutathera European Public Assessment Report. EMA/506460/2017. London: European Medicines Agency;2017.
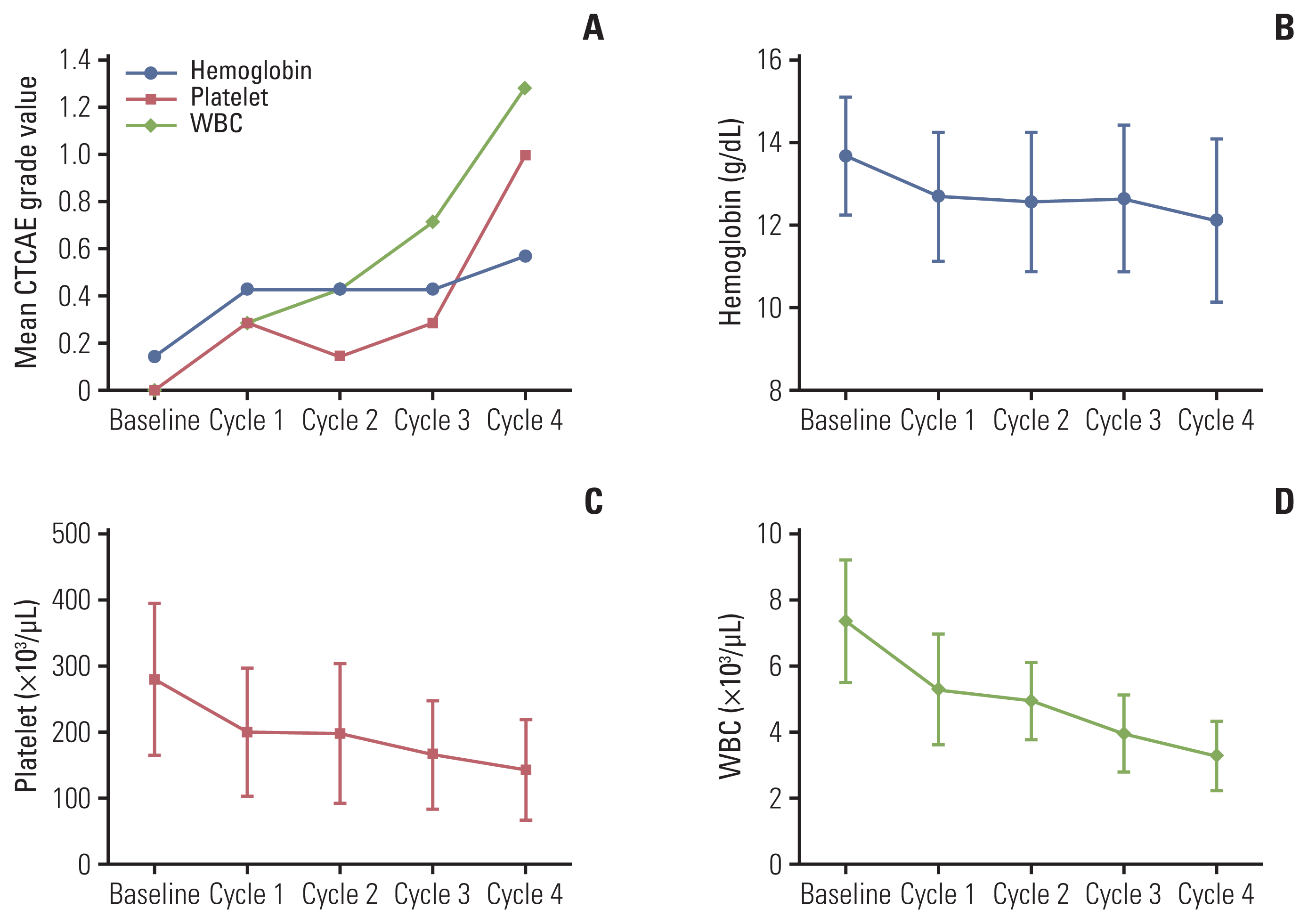
18. Ciesla B. Hematology in practice. 2nd ed. Philadelphia, PA: F.A. Davis Company;2012.
19. Heylmann D, Rodel F, Kindler T, Kaina B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim Biophys Acta. 2014; 1846:121–9.
20. Kong G, Grozinsky-Glasberg S, Hofman MS, Callahan J, Meirovitz A, Maimon O, et al. Efficacy of peptide receptor radionuclide therapy for functional metastatic paraganglioma and pheochromocytoma. J Clin Endocrinol Metab. 2017; 102:3278–87.
21. Gains JE, Bomanji JB, Fersht NL, Sullivan T, D’Souza D, Sullivan KP, et al. 177Lu-DOTATATE molecular radiotherapy for childhood neuroblastoma. J Nucl Med. 2011; 52:1041–7.
22. Marincek N, Radojewski P, Dumont RA, Brunner P, Muller-Brand J, Maecke HR, et al. Somatostatin receptor-targeted radiopeptide therapy with 90Y-DOTATOC and 177Lu-DOTATOC in progressive meningioma: long-term results of a phase II clinical trial. J Nucl Med. 2015; 56:171–6.
23. Parghane RV, Naik C, Talole S, Desmukh A, Chaukar D, Banerjee S, et al. Clinical utility of (177) Lu-DOTATATE PRRT in somatostatin receptor-positive metastatic medullary carcinoma of thyroid patients with assessment of efficacy, survival analysis, prognostic variables, and toxicity. Head Neck. 2020; 42:401–16.
24. Zidan L, Iravani A, Oleinikov K, Ben-Haim S, Gross DJ, Meirovitz A, et al. Efficacy and safety of (177)Lu-DOTATATE in lung neuroendocrine tumors: a bicenter study. J Nucl Med. 2022; 63:218–25.
25. Giuffrida G, Ferrau F, Laudicella R, Cotta OR, Messina E, Granata F, et al. Peptide receptor radionuclide therapy for aggressive pituitary tumors: a monocentric experience. Endocr Connect. 2019; 8:528–35.
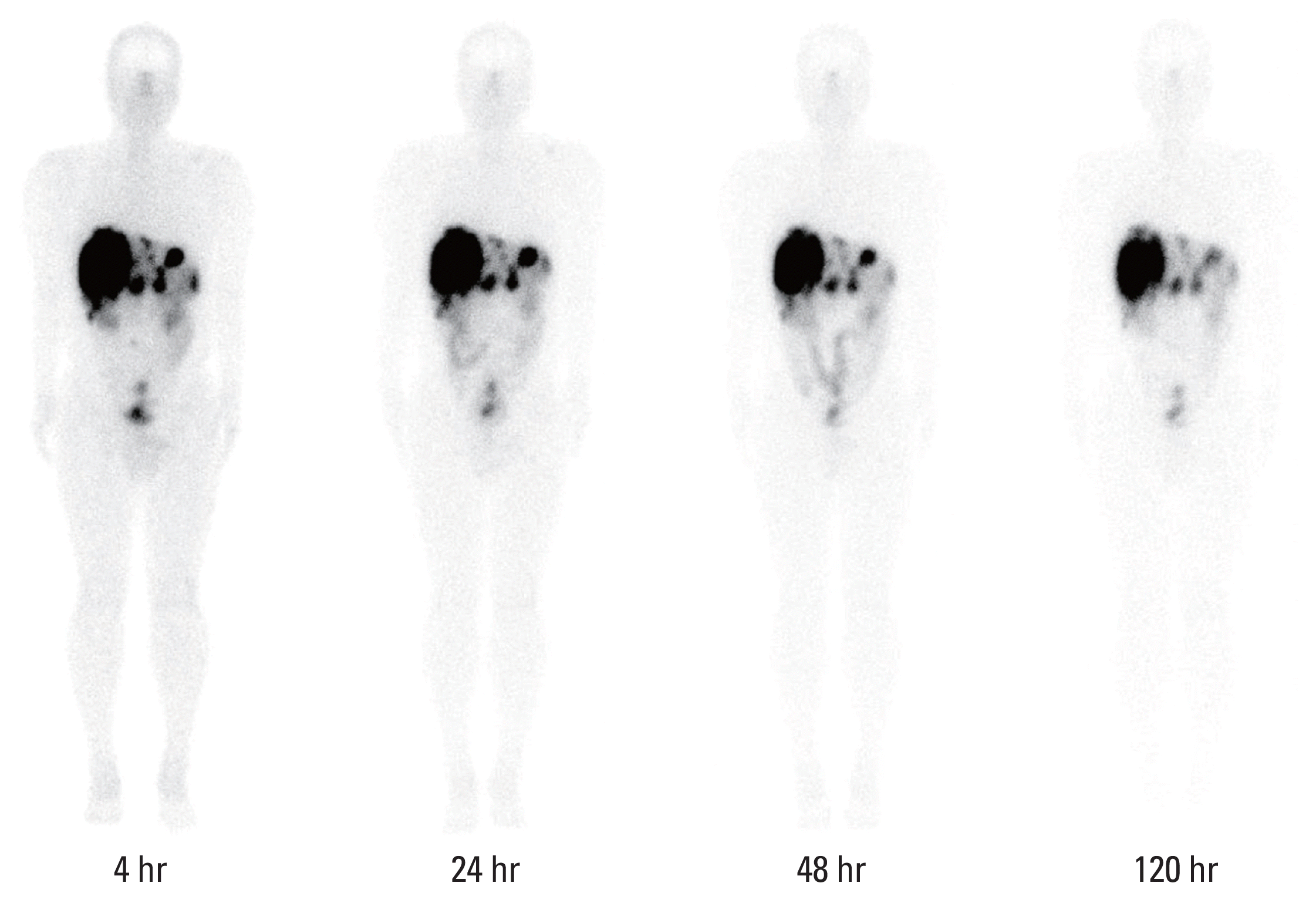
26. Lee MS, Kim JH, Paeng JC, Kang KW, Jeong JM, Lee DS, et al. Whole-body voxel-based personalized dosimetry: the multiple voxel S-value approach for heterogeneous media with nonuniform activity distributions. J Nucl Med. 2018; 59:1133–9.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download