Abstract
Purpose
Materials and Methods
Results
Electronic Supplementary Material
Notes
Ethical Statement
The KALC was approved by the Institutional Review Board (IRB) of the National Cancer Center (approval number: NCC2018-0193). The requirement for informed consent was waived by the IRB due to the retrospective nature of the study. All methods were conducted in accordance with the relevant guidelines.
Author Contributions
Conceived and designed the analysis: Jeon DS, Kim HC, Choi CM.
Collected the data: Jeon DS, Kim HC, Kim TJ, Kim HK, Moon MH, Beck KS, Suh YG, Song C, Ahn JS, Lee JE, Lim JU, Jeon JH, Jung KW, Jung CY, Cho JS, Choi YD, Hwang SS, Choi CM.
Contributed data or analysis tools: Jeon DS, Kim HC, Kim SH, Kim TJ, Kim HK, Moon MH, Beck KS, Suh YG, Song C, Ahn JS, Lee JE, Lim JU, Jeon JH, Jung KW, Jung CY, Cho JS, Choi YD, Hwang SS, Choi CM.
Performed the analysis: Jeon DS, Kim HC, Kim SH, Choi CM.
Wrote the paper: Jeon DS, Kim HC, Choi CM.
Acknowledgments
References
Fig. 1
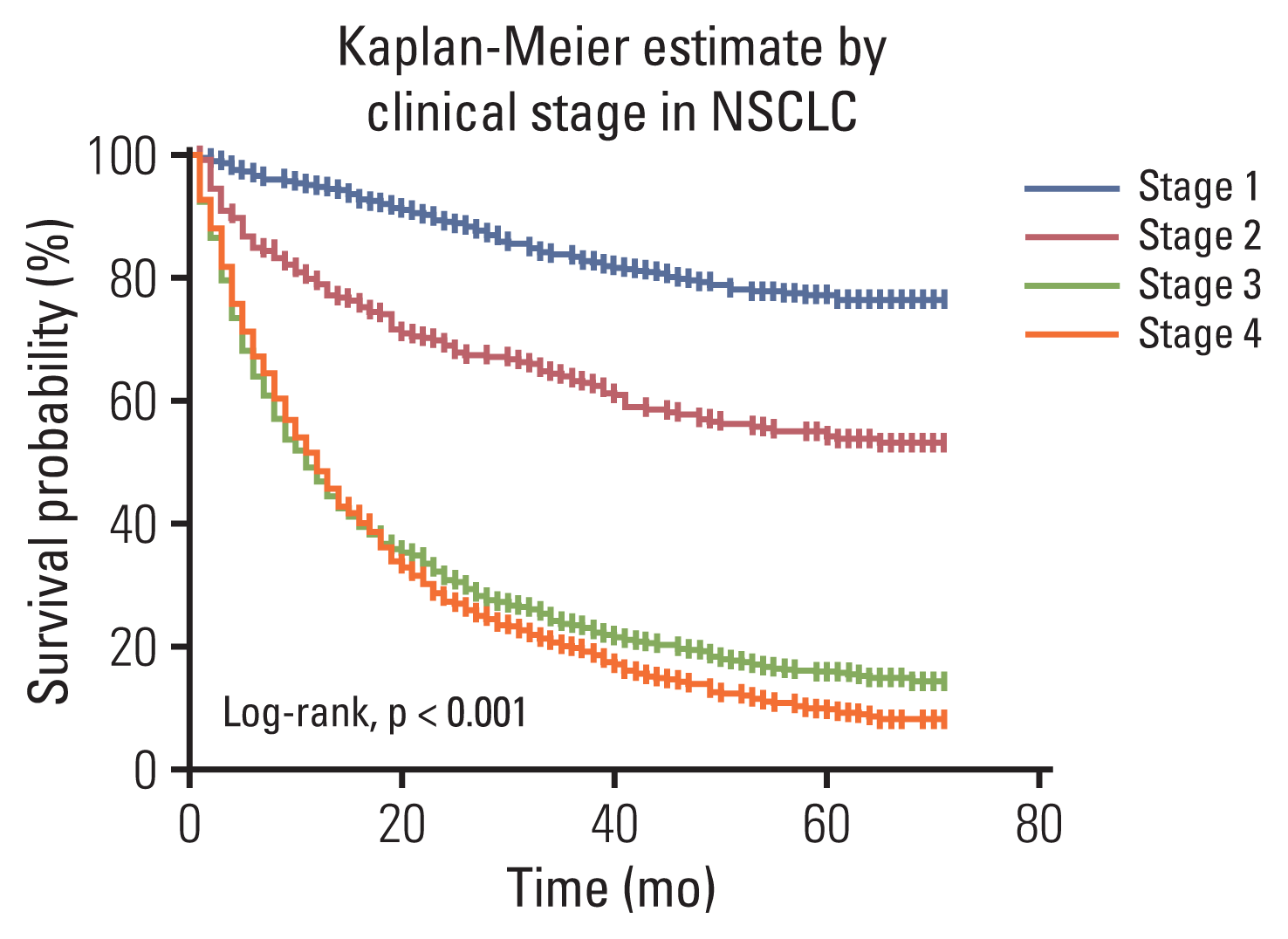
Fig. 2
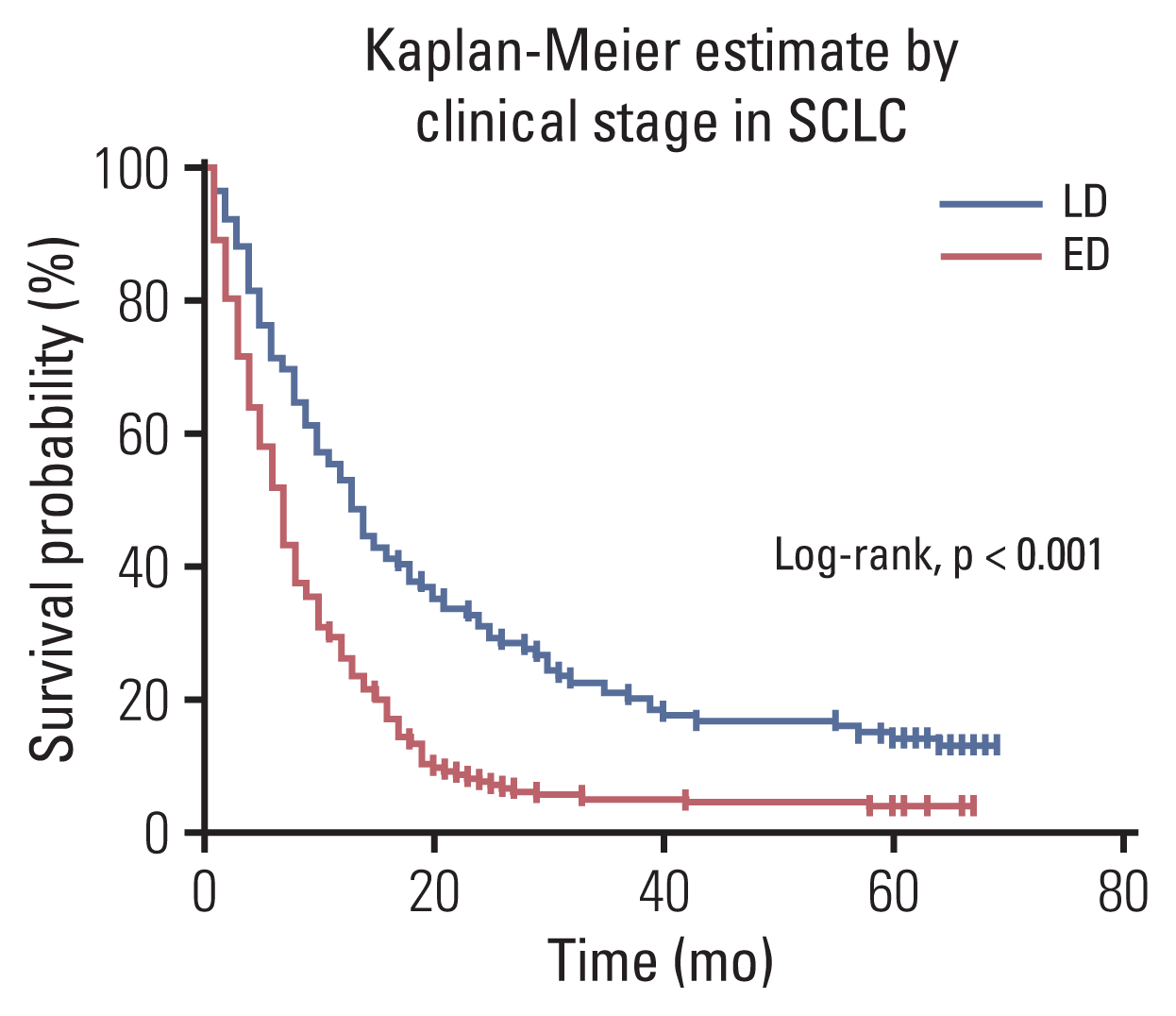
Table 1
Table 2
Table 3
Table 4
Table 5
| Stage | Time (yr) | No. at risk | No. of deaths | Observed survival ratea) | Expected survival rateb) | Relative survival (95% CI) |
|---|---|---|---|---|---|---|
| 1 | 0.0–1.0 | 390 | 19 | 0.95 | 0.99 | 0.96 (0.93–0.98) |
| 1.0–2.0 | 371 | 22 | 0.89 | 0.98 | 0.91 (0.88–0.94) | |
| 2.0–3.0 | 349 | 22 | 0.84 | 0.97 | 0.87 (0.82–0.90) | |
| 3.0–4.0 | 327 | 16 | 0.80 | 0.96 | 0.83 (0.79–0.87) | |
| 4.0–5.0 | 311 | 9 | 0.78 | 0.94 | 0.82 (0.77–0.86) | |
| 2 | 0.0–1.0 | 261 | 54 | 0.79 | 0.98 | 0.80 (0.75–0.85) |
| 1.0–2.0 | 207 | 26 | 0.69 | 0.97 | 0.72 (0.65–0.77) | |
| 2.0–3.0 | 181 | 15 | 0.64 | 0.95 | 0.67 (0.60–0.73) | |
| 3.0–4.0 | 166 | 16 | 0.57 | 0.94 | 0.61 (0.55–0.67) | |
| 4.0–5.0 | 150 | 7 | 0.55 | 0.92 | 0.59 (0.53–0.66) | |
| 3 | 0.0–1.0 | 653 | 349 | 0.47 | 0.98 | 0.47 (0.43–0.51) |
| 1.0–2.0 | 304 | 105 | 0.30 | 0.97 | 0.31 (0.28–0.35) | |
| 2.0–3.0 | 199 | 52 | 0.23 | 0.96 | 0.24 (0.20–0.27) | |
| 3.0–4.0 | 147 | 27 | 0.18 | 0.94 | 0.20 (0.16–0.23) | |
| 4.0–5.0 | 120 | 21 | 0.15 | 0.92 | 0.16 (0.14–0.19) | |
| 4 | 0.0–1.0 | 514 | 262 | 0.49 | 0.98 | 0.50 (0.45–0.54) |
| 1.0–2.0 | 252 | 112 | 0.27 | 0.97 | 0.28 (0.24–0.32) | |
| 2.0–3.0 | 140 | 42 | 0.19 | 0.95 | 0.20 (0.17–0.24) | |
| 3.0–4.0 | 98 | 30 | 0.13 | 0.94 | 0.14 (0.11–0.17) | |
| 4.0–5.0 | 68 | 19 | 0.10 | 0.92 | 0.10 (0.08–0.13) |
Table 6
| Stage | Time (yr) | No. at risk | No. of deaths | Observed survival ratea) | Expected survival rateb) | Relative survival (95% CI) |
|---|---|---|---|---|---|---|
| LD | 0.0–1.0 | 122 | 56 | 0.54 | 0.98 | 0.55 (0.49–0.64) |
| 1.0–2.0 | 66 | 27 | 0.32 | 0.97 | 0.33 (0.25–0.42) | |
| 2.0–3.0 | 39 | 14 | 0.20 | 0.96 | 0.21 (0.14–0.29) | |
| 3.0–4.0 | 25 | 5 | 0.16 | 0.95 | 0.17 (0.11–0.25) | |
| 4.0–5.0 | 20 | 2 | 0.15 | 0.94 | 0.16 (0.10–0.23) | |
| ED | 0.0–1.0 | 213 | 156 | 0.27 | 0.98 | 0.27 (0.21–0.33) |
| 1.0–2.0 | 57 | 41 | 0.08 | 0.97 | 0.08 (0.05–0.12) | |
| 2.0–3.0 | 16 | 6 | 0.05 | 0.96 | 0.05 (0.03–0.08) | |
| 3.0–4.0 | 10 | 1 | 0.04 | 0.95 | 0.04 (0.02–0.08) | |
| 4.0–5.0 | 9 | 1 | 0.04 | 0.95 | 0.04 (0.02–0.07) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download