1. Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006; 21(Suppl 14):S290–S304. PMID:
16892449.
2. Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006; 355(9):896–908. PMID:
16943402.
3. Schüpbach MW, Welter ML, Bonnet AM, Elbaz A, Grossardt BR, Mesnage V, et al. Mortality in patients with Parkinson’s disease treated by stimulation of the subthalamic nucleus. Mov Disord. 2007; 22(2):257–261. PMID:
17149702.
4. Toft M, Lilleeng B, Ramm-Pettersen J, Skogseid IM, Gundersen V, Gerdts R, et al. Long-term efficacy and mortality in Parkinson’s disease patients treated with subthalamic stimulation. Mov Disord. 2011; 26(10):1931–1934. PMID:
21656853.
5. Rocha S, Monteiro A, Linhares P, Chamadoira C, Basto MA, Reis C, et al. Long-term mortality analysis in Parkinson’s disease treated with deep brain stimulation. Parkinsons Dis. 2014; 2014:717041. PMID:
24772365.
6. Bang Henriksen M, Johnsen EL, Sunde N, Vase A, Gjelstrup MC, Østergaard K. Surviving 10 years with deep brain stimulation for Parkinson’s disease--a follow-up of 79 patients. Eur J Neurol. 2016; 23(1):53–61. PMID:
25492023.
7. Ryu HS, Kim MS, You S, Kim MJ, Kim YJ, Kim J, et al. Mortality of advanced Parkinson’s disease patients treated with deep brain stimulation surgery. J Neurol Sci. 2016; 369:230–235. PMID:
27653896.
8. Ngoga D, Mitchell R, Kausar J, Hodson J, Harries A, Pall H. Deep brain stimulation improves survival in severe Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2014; 85(1):17–22. PMID:
23843542.
9. Wider C, Pollo C, Bloch J, Burkhard PR, Vingerhoets FJ. Long-term outcome of 50 consecutive Parkinson’s disease patients treated with subthalamic deep brain stimulation. Parkinsonism Relat Disord. 2008; 14(2):114–119. PMID:
17822940.
10. Lilleeng B, Brønnick K, Toft M, Dietrichs E, Larsen JP. Progression and survival in Parkinson’s disease with subthalamic nucleus stimulation. Acta Neurol Scand. 2014; 130(5):292–298. PMID:
24495107.
11. Genc G, Abboud H, Oravivattanakul S, Alsallom F, Thompson NR, Cooper S, et al. Socioeconomic status may impact functional outcome of deep brain stimulation surgery in Parkinson’s disease. Neuromodulation. 2016; 19(1):25–30. PMID:
26076401.
12. Merola A, Rizzi L, Artusi CA, Zibetti M, Rizzone MG, Romagnolo A, et al. Subthalamic deep brain stimulation: clinical and neuropsychological outcomes in mild cognitive impaired parkinsonian patients. J Neurol. 2014; 261(9):1745–1751. PMID:
24952619.
14. Statistics Korea. The 8th Korean Standard Disease Cause Classification. Daejeon, Korea: Statistics Korea;2020.
15. Jeong SM, Han K, Kim D, Rhee SY, Jang W, Shin DW. Body mass index, diabetes, and the risk of Parkinson’s disease. Mov Disord. 2020; 35(2):236–244. PMID:
31785021.
16. Nam GE, Kim NH, Han K, Choi KM, Chung HS, Kim JW, et al. Chronic renal dysfunction, proteinuria, and risk of Parkinson’s disease in the elderly. Mov Disord. 2019; 34(8):1184–1191. PMID:
31021467.
17. York MK, Dulay M, Macias A, Levin HS, Grossman R, Simpson R, et al. Cognitive declines following bilateral subthalamic nucleus deep brain stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008; 79(7):789–795. PMID:
17965146.
18. Shin H, Shin Y, Hwang D, Choi B, Yoon S. Medical Aid System Evaluation and Basic Plan Establishment Research. Sejong, Korea: Korea Institute for Health and Social Affairs;2017.
19. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004; 57(12):1288–1294. PMID:
15617955.
20. Shintani AK, Girard TD, Eden SK, Arbogast PG, Moons KG, Ely EW. Immortal time bias in critical care research: application of time-varying Cox regression for observational cohort studies. Crit Care Med. 2009; 37(11):2939–2945. PMID:
19770737.
21. Herlofson K, Lie SA, Arsland D, Larsen JP. Mortality and Parkinson disease: a community based study. Neurology. 2004; 62(6):937–942. PMID:
15037696.
22. Willis AW, Schootman M, Kung N, Evanoff BA, Perlmutter JS, Racette BA. Predictors of survival in patients with Parkinson disease. Arch Neurol. 2012; 69(5):601–607. PMID:
22213411.
23. Hughes TA, Ross HF, Mindham RH, Spokes EG. Mortality in Parkinson’s disease and its association with dementia and depression. Acta Neurol Scand. 2004; 110(2):118–123. PMID:
15242420.
24. Harris-Hayes M, Willis AW, Klein SE, Czuppon S, Crowner B, Racette BA. Relative mortality in U.S. Medicare beneficiaries with Parkinson disease and hip and pelvic fractures. J Bone Joint Surg Am. 2014; 96(4):e27. PMID:
24553896.
25. Benzinger P, Rapp K, Maetzler W, König HH, Jaensch A, Klenk J, et al. Risk for femoral fractures in Parkinson’s disease patients with and without severe functional impairment. PLoS One. 2014; 9(5):e97073. PMID:
24853110.
26. Lau B, Meier N, Serra G, Czernecki V, Schuepbach M, Navarro S, et al. Axial symptoms predict mortality in patients with Parkinson disease and subthalamic stimulation. Neurology. 2019; 92(22):e2559–e2570. PMID:
31043471.
27. Weaver FM, Stroupe KT, Smith B, Gonzalez B, Huo Z, Cao L, et al. Survival in patients with Parkinson’s disease after deep brain stimulation or medical management. Mov Disord. 2017; 32(12):1756–1763. PMID:
29150873.
28. Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol. 2011; 68(12):1550–1556. PMID:
21825213.
29. Zhang Y, Wang C, Wang Y, Xiao Q, Liu J, Ma J, et al. Mortality from Parkinson’s disease in China: findings from a ten-year follow up study in Shanghai. Parkinsonism Relat Disord. 2018; 55:75–80. PMID:
29802079.
30. Rocha AL, Oliveira A, Sousa C, Monteiro P, Rosas MJ, Vaz R. Long term mortality of patients with Parkinson’s disease treated with deep brain stimulation in a reference center. Clin Neurol Neurosurg. 2021; 202:106486. PMID:
33493881.
31. Kanna SV, Bhanu K. A simple bedside test to assess the swallowing dysfunction in Parkinson’s disease. Ann Indian Acad Neurol. 2014; 17(1):62–65. PMID:
24753662.
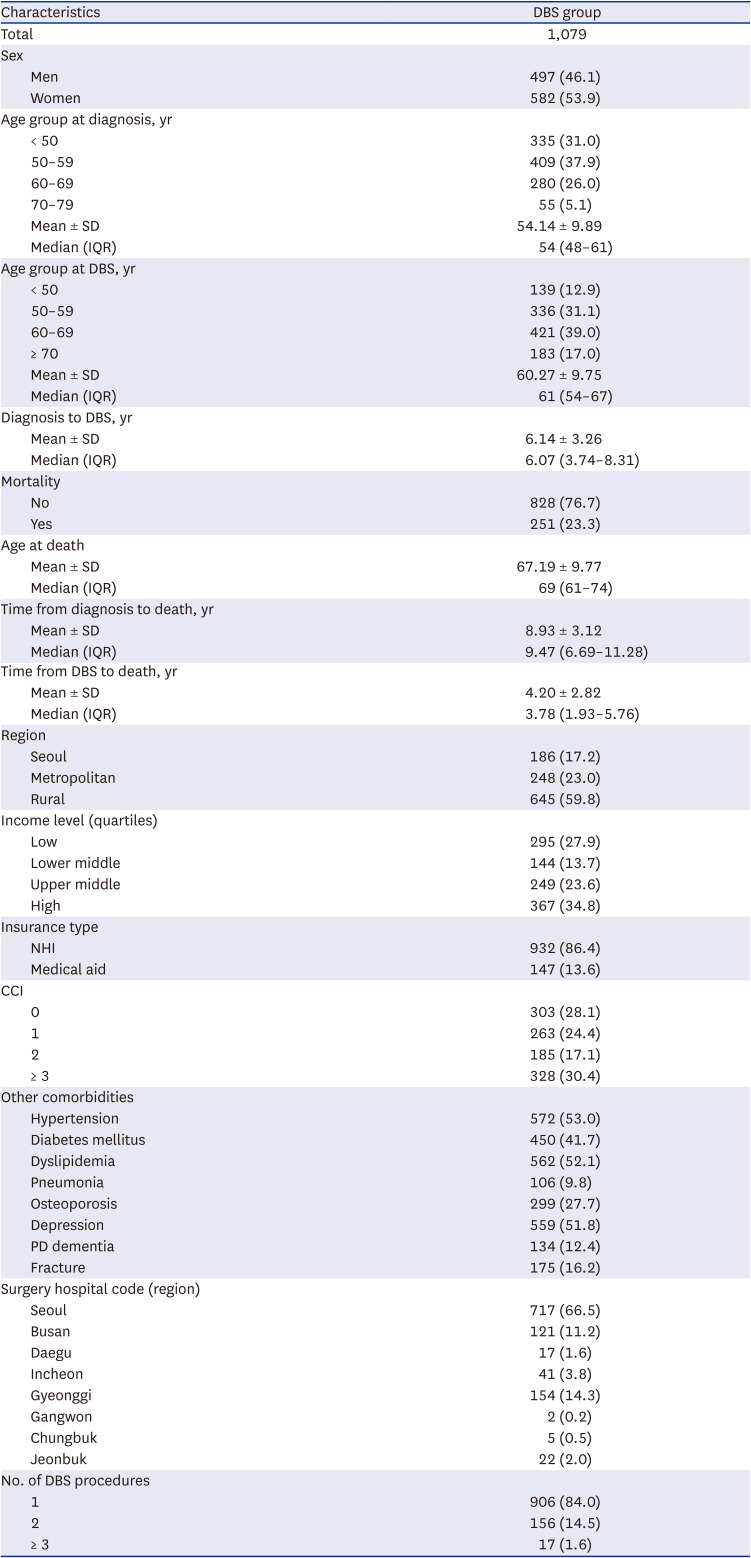
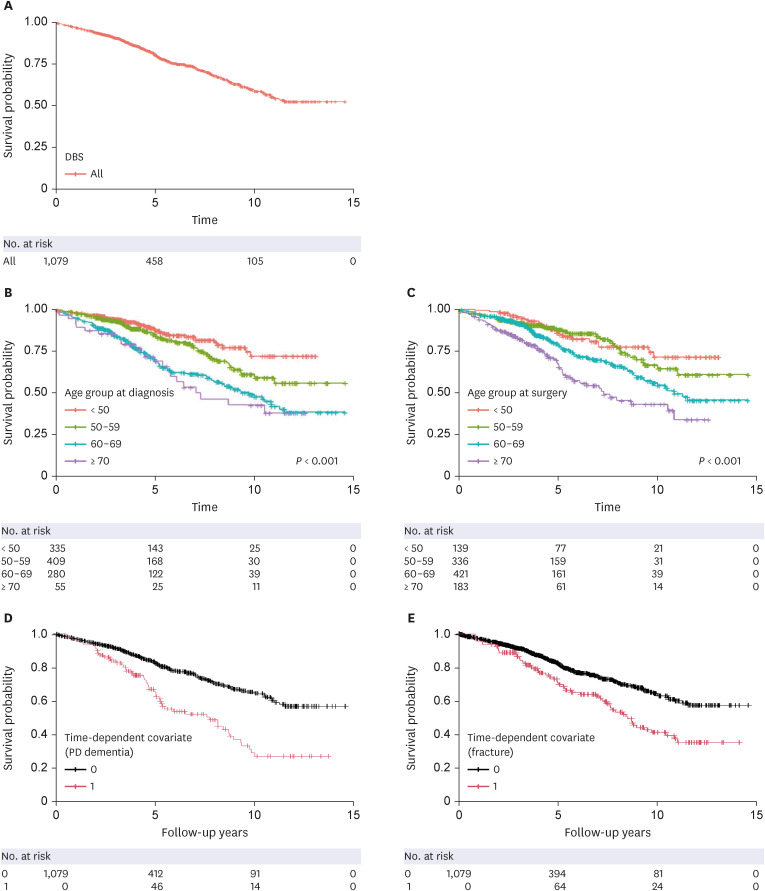
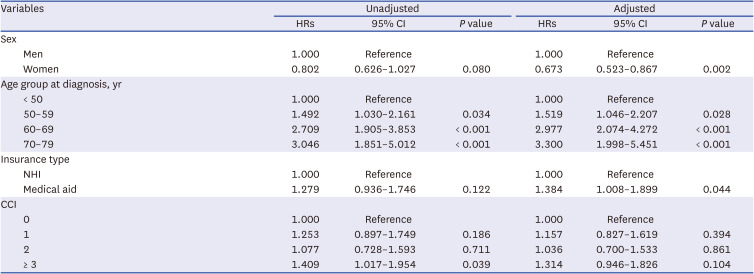
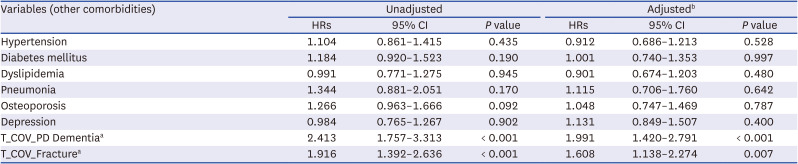




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download