Abstract
Purpose
This study aims to investigate the feasibility of Zenith Fenestrated AAA Endovascular Graft (Z-FEN, Cook Medical) from a single Korean institution database by evaluating the vascular anatomy of Korean abdominal aortic aneurysm (AAA) patients with hostile aortic neck.
Methods
This is a retrospective study on patients with AAA who underwent endovascular aortic repair (EVAR) and open surgery repair between January 2012 and December 2021 (n = 211). The anatomic characteristics of the aortic neck were evaluated using 3-dimensional reconstructed computed tomographic scans. For the juxtarenal AAA patients (n = 39), feasibility of fenestrated stent graft was evaluated under the protocol of fenestrated EVAR. For those who were not suitable for the application of Z-FEN, the reasons for unsuitability were analyzed.
Results
Among 211 AAA patients, 108 patients (51.2%) had complex aortic neck, and 39 (18.5%) had insufficient aortic neck length (<15 mm) for conventional EVAR. Of the 39 patients with juxtarenal AAAs, 13 (33.3%) were determined feasible for Z-FEN. Twenty-six patients (66.7%) were noncandidate for Z-FEN due to severe neck angulation, short aortic neck length, inadequate iliac artery anatomy, large aortic neck diameter, and severe calcification and thrombosis. Proximal aortic neck length of the non-feasible group was significantly shorter than that of the feasible group (P = 0.002).
Go to : 
Currently, endovascular aortic repair (EVAR) is the first treatment option in patients with abdominal aortic aneurysms (AAA) who have appropriate vascular anatomy [1]. Aortic neck length, angulation, and morphology are assessed for the suitability of EVAR, as it is essential to secure enough infrarenal space for fixation of the endograft. A previous study has reported that as aortic aneurysmal diseases progress, they move up cranially in the aorta [2], and this indicates that all AAA patients could potentially turn up having an unfavorable aortic neck, making it hard to apply conventional EVAR. Fenestrated EVAR (FEVAR) was one of the techniques developed to keep blood flow through the subsidiary arteries while securing the proximal fixation zone in patients with inadequate aortic neck.
Since its first use in 1998, clinical studies supporting the use of FEVAR were reported [34], and, currently, FEVAR is recommended over open repair in patients with juxtarenal aortic aneurysms according to the most recent practice guidelines published by the European Society for Vascular Surgery [5]. Zenith Fenestrated AAA Endovascular Graft (Z-FEN; Cook Medical, Brisbane, QLD, Australia) was approved by the U.S. Food and Drug Administration in 2012 and has been used worldwide ever since. According to the 5-year follow-up study involving 14 medical centers in the United States with the results of 67 patients who were treated with Z-FEN, freedom from all-cause mortality was 88.8% ± 4.2% and freedom from aneurysm-related mortality was 96.8% ± 2.3% [6]. Also, low rates of type 1A endoleak, aneurysmal sac enlargement, and device migration were reported during the 5-year follow-up [67]. These low rates of stent failure support the use of Z-FEN. Z-FEN is not yet applicable in Korea, but it will be available soon, so it is necessary to study the feasibility of Z-FEN in juxtarenal AAA patients.
This study aims to investigate the feasibility of Z-FEN on the Korean population by evaluating the vascular anatomy of Korean AAA patients with hostile aortic neck. This process requires measurements of anatomical components of the AAAs, and the authors expect that the measurements acquired in this study can be used as a basis for the development of an off-the-shelf device that can be applied to most Koreans. Off-the-shelf devices can reduce the limitations of a custom-made device, such as high costs and treatment delay, and will allow the widespread application of Z-FEN.
Go to : 
This was a retrospective study. AAA patients who underwent EVAR and open surgery repair between January 2012 to December 2021 were included in the study (n = 211). CT scans were performed in all patients preoperatively and angiography obtained at the physician’s discretion was used to evaluate the anatomical characteristics of the AAA. Aquarius iNtuition software (TeraRecon, Foster City, CA, USA) was used to reconstruct the images 3-dimensionally (3D). We used Society for Vascular Surgery (SVS) reporting standards to determine the morphological measurements of the AAAs [8]. These include aortic neck length, aortic neck diameter, suprarenal aortic neck angle, infrarenal aortic neck angle, degree of thrombus and calcification of the aortic neck, maximum aneurysm diameter, degree of aneurysm thrombus and calcification, common iliac artery diameter and length, and external iliac artery diameter. The measurements were entered into a database used for this study, which was approved by the Institutional Review Board of The Catholic University of Korea, Seoul St. Mary’s Hospital (No. KC22RASI0439). This study was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature.
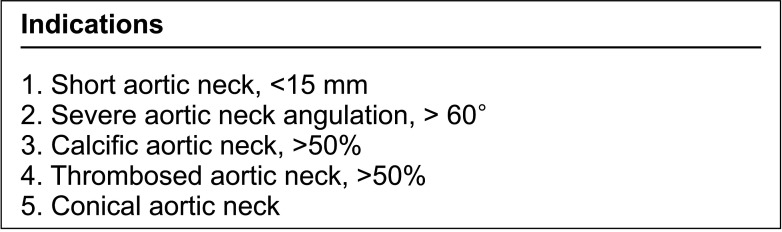
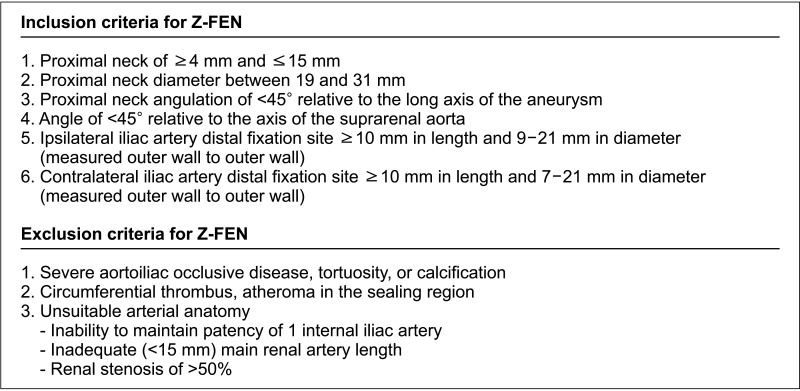
Indications for complex aortic neck are shown in Fig. 1. Patients with aortic neck satisfying the anatomical characteristics of a complex neck were included in the complex aortic neck group (n = 108), and they were evaluated for the feasibility of FEVAR. The feasibility was assessed through 2 steps. First, the patients were checked if they had sufficient aortic neck length for the application of conventional EVAR. Aortic neck lengths shorter than 15 mm were included in the juxtarenal AAA group and were further evaluated. Secondly, the patients were checked whether they met the anatomical inclusion or exclusion criteria described in Fig. 2. Aortic neck and iliac artery anatomy, neck angulation, degree of calcification and thrombosis were taken into account, which were defined according to the SVS reporting standards [8]. For those who passed both steps, actual stent-graft planning and sizing of Z-FEN were performed, and incorporated target vessels, device design, and fenestration types were analyzed. Anatomical characteristics of the Z-FEN applicable patients were also analyzed. For those who were not suitable for the application of Z-FEN, the reasons for unsuitability were evaluated. Anatomical characteristics were compared between the FEVAR feasible group and the unfeasible group. The actual treatments performed in both Z-FEN feasible group and the non-feasible group were also analyzed. Categorical variables were presented as relative frequencies (percent), while continuous variables were expressed as means and standard deviations. PASW Statistics ver. 18.0 (IBM Corp., Armonk, NY, USA) was used for data analysis.
Z-FEN consists of a 2-piece main body (proximal body graft with fenestration and distal bifurcated body graft) and contralateral limb, with fenestrations and/or scallops for the renal and mesenteric arteries. The upper part of the proximal body is composed of custom fenestrations to fit the diverse anatomical variations of the positions of the renal arteries and the superior mesenteric artery (SMA) in relation to the aorta. The suprarenal fixating anchoring barbs superior to the custom fenestrations add stability to the graft-to-vessel attachment.
The planning and sizing of the Z-FEN is the most important step toward the successful placement of the graft. It requires a thorough anatomical analysis of the abdominal aorta and its subsidiary branches. CT scans were used to obtain the anatomical data needed, and post-processing software is desirable as it supports planar reformatting, allowing more accurate measurements. In this study, Aquarius iNtuition software was used.
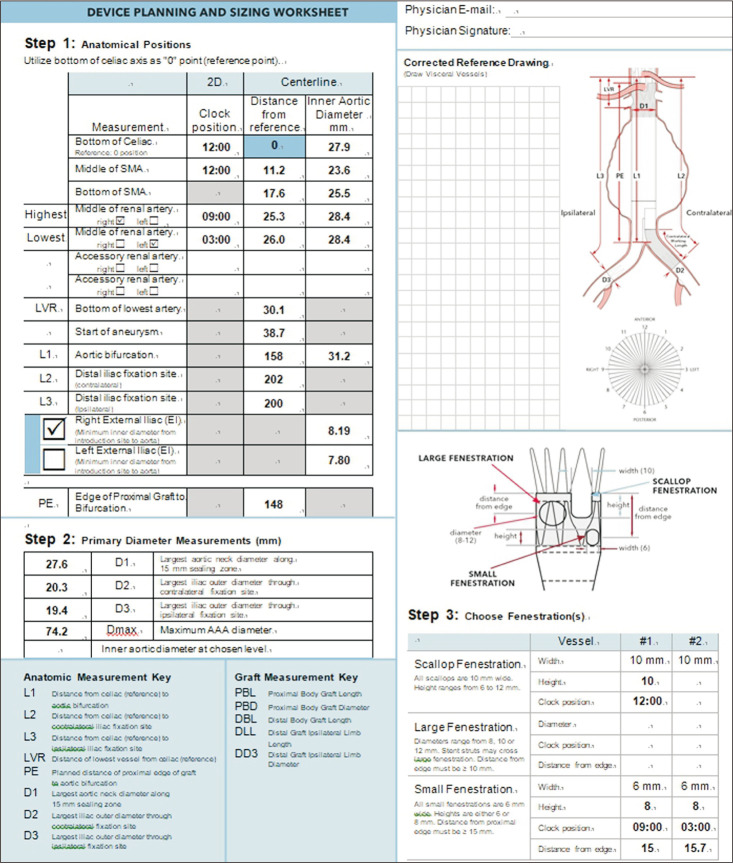
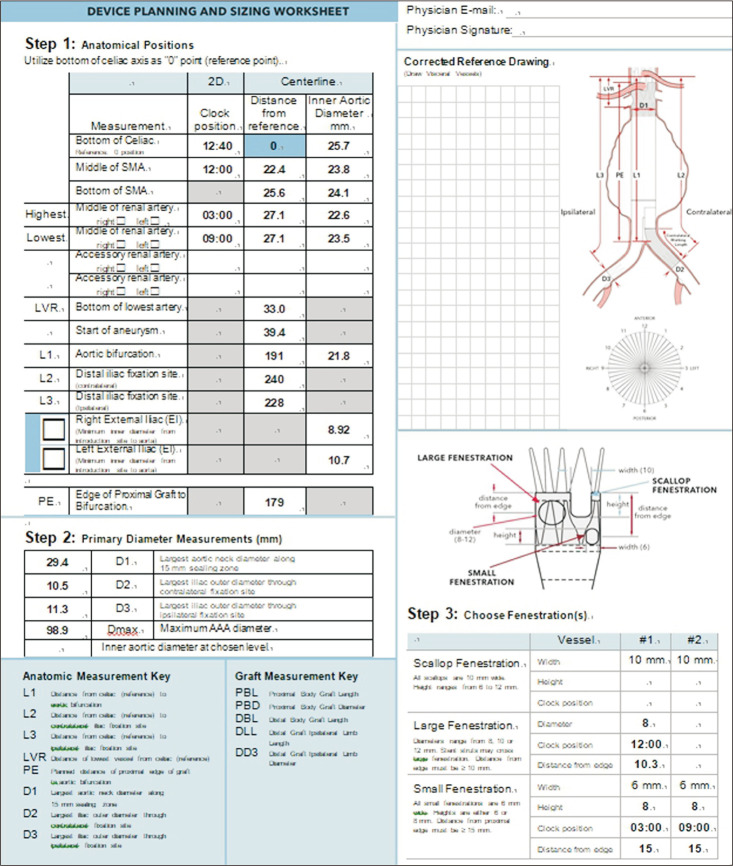
The first step was setting an aortic centerline for subsequent measurements. Using 3D reconstruction software, the centerline was adjusted to reflect the way in which the stent graft was anticipated to be inserted into the aorta. After setting the centerline, in a straightened view of the aorta, the celiac trunk, SMAs, renal arteries, the aortic bifurcation, and each iliac artery bifurcations were marked in order (Fig. 3). The next step was to determine the proximal edge of the endograft. The instructions for use (IFU) require a minimum of 1.5 cm as an acceptable proximal sealing zone, but the authors used 2 cm of healthy parallel-walled aorta to be more conservative. A distance of 2 cm proximal to the start of aneurysm is marked, and a distance of 1.5 cm proximal to the highest point of the renal arteries is also marked. This is to follow the Z-FEN graft specification rule that states the first small fenestration must be more than 15 mm apart from the top of the fabric. The higher of the 2 marked points would be the proximal edge of the graft.
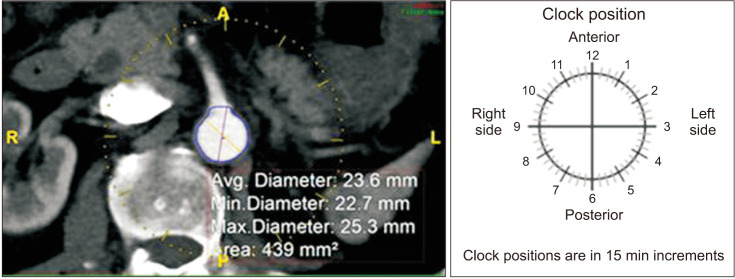
Once the proximal edge was determined, the required diameters, lengths, and clock positions of the target arteries were measured. The diameters of the proximal sealing zone and distal sealing zones in each common iliac artery, and the lengths from the proximal edge to each visceral branch, aortic bifurcation, and each iliac bifurcation were measured. Clock positions of the branching arteries were assessed using the axial views of straightened multiplanar reformatting mode (Fig. 4) and were rounded to the nearest 15-minute interval. For scallop and fenestration design, if the SMA is within 12 mm from the proximal edge, it would be incorporated using a scallop, and if it is located farther than 12 mm from the proximal edge, a large fenestration would be used. For renal arteries, small fenestrations with a height of 8 mm were chosen. After acquiring all the anatomical data regarding vascular anatomy, the length of the proximal component (L1), distal bifurcated component (L2), and iliac limbs (L3, L4) were calculated by putting in the anatomical measurements obtained above to the preset formulas, and the parts of the stent graft were chosen accordingly. For the proximal sealing, a 2-proximal-seal-stent design was chosen routinely.
The feasibility of Z-FEN was assessed through the clinician’s predictions of delivering the potential endografts and securing the fixation at the proximal aortic neck and distal iliac arteries. The iliac arteries were also evaluated for whether they could deliver both the main body and the contralateral limb extensions, to determine whether they were suitable as access vessels. A total of 3 vascular surgeons with more than 10 years of experience with the EVAR procedure took part in the process of assessing the feasibility. One vascular surgeon completed the planning and sizing of the stent graft using the anatomical data acquired, and the other 2 vascular surgeons confirmed the results.
Go to : 
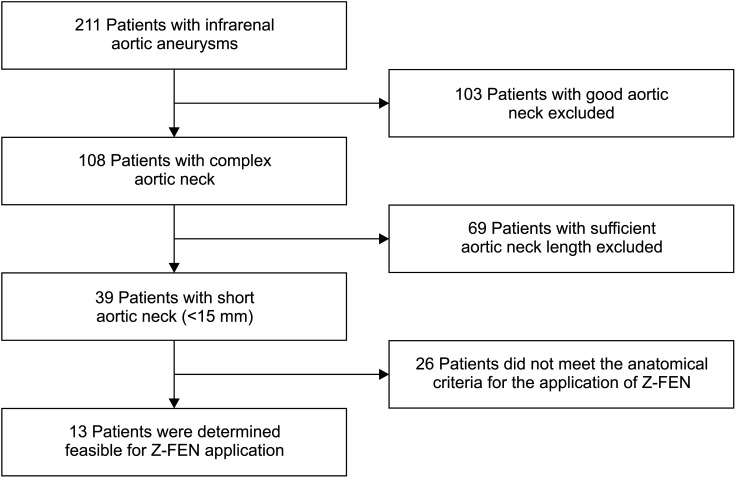
Among a total of 211 infrarenal AAA patients, 108 patients (51.2%) were determined to have a complex aortic neck, and 39 patients (18.5%) had insufficient aortic neck length (<15 mm) for conventional EVAR. Thirty-nine patients who were allocated into juxtarenal AAA group were assessed for the application of Z-FEN, and 13 patients (33.3%) were determined feasible for Z-FEN (Fig. 5). Twenty-six patients (66.7%) were noncandidates for Z-FEN due to severe neck angulation, short aortic neck length (<4 mm), inadequate common iliac artery anatomy, large aortic neck diameter (>31 mm), and severe calcification and thrombosis. There were patients who had more than 1 reason for unsuitability, and they were all taken into account separately in the data analysis. The reasons for unsuitability are analyzed in Table 1. The most common criterion outside of IFU was severe neck angulation, which was expressed in 53.8% of the patients who were not applicable. Other exclusion criteria include aortic neck of less than 4 mm in 10 patients (38.5%), too large (>21 mm) or too narrow (<9 mm) common iliac artery diameters in 9 patients (34.6%), aortic neck diameters larger than 31 mm in 5 patients (19.2%), and severe aortic neck calcification and thrombosis in 4 and 2 patients, respectively.
Comparisons of the anatomical measurements between the Z-FEN feasible group (n = 13) and the non-feasible group (n = 26) are shown in Table 2. Proximal aortic neck length of the non-feasible group was significantly shorter than that of the feasible group (P = 0.002), and severe neck angulations were only present in the non-feasible group (P = 0.001).
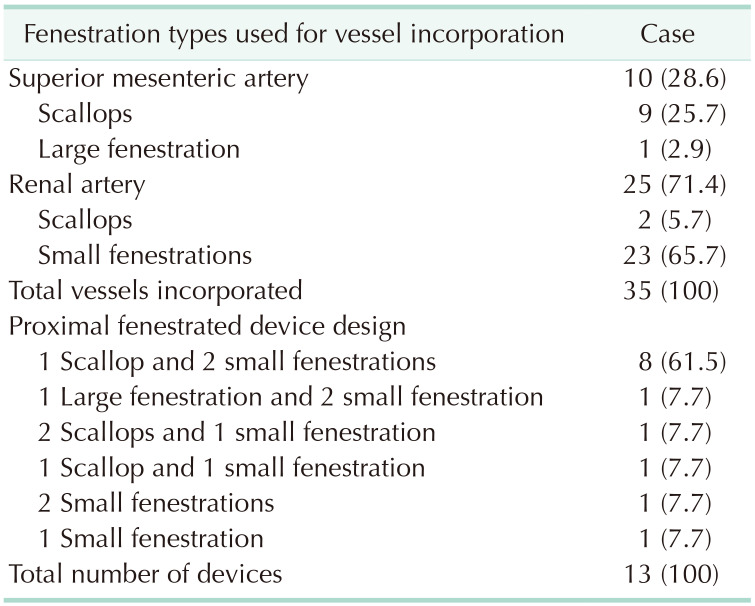
Stent-graft planning and sizing were performed for the 13 patients who had AAAs viable for Z-FEN, and incorporated target vessels, fenestration types and designs are presented in Table 3. Including 10 SMAs and 25 renal arteries, a total of 35 target vessels were incorporated. The most common device design was a scallop for SMA and 2 small fenestrations for renal arteries which covered 61.5% of the patients who were Z-FEN applicable. A typical device planning and sizing worksheet of the most common design is shown in Fig. 6, and that of the device design which consists of 1 large fenestration and 2 small fenestrations is shown in Fig. 7. With enough distance from the proximal edge, large fenestration is used to accommodate SMA, whereas a scallop is used if the distance from the proximal edge is less than 10 mm.
Anatomical characteristics of the Z-FEN applicable patients are presented in Table 4. The mean maximum aneurysm diameter was 63.8 ± 14.7 mm (range, 46.1–98.9 mm), and the diameter of the largest aortic neck along the proximal sealing zone averaged 26.9 ± 3.4 mm (range, 19.7–30.2 mm).
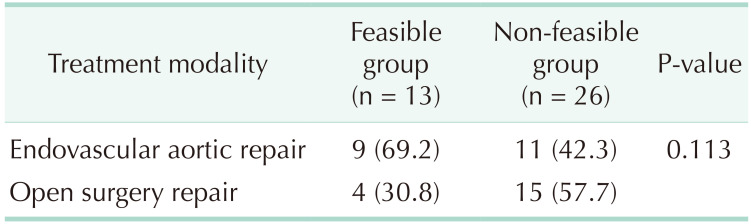
The treatments that were actually given to patients in both Z-FEN feasible and non-feasible groups are shown in Table 5. In Z-FEN feasible group, 69.2% of the patients were treated with EVAR, whereas 42.3% of the patients in the non-feasible group received EVAR. Although there was no statistically significant difference, fewer open surgeries were performed in Z-FEN feasible group compared to the non-feasible group (P = 0.113).
Go to : 
Conventional EVAR provides limited coverage for AAA patients. In a retrospective analysis, 52% of AAA patients were deemed unsuitable for conventional EVAR due to complex aortic neck anatomy, and application of EVAR outside the IFU resulted in higher complication rates [9]. EVAR eligibility in Korean patients for standard and extended IFUs was reported to be 37.5% and 55.1% in men, and 11.3% and 25.4% in women, respectively [10]. Overall eligibilities for IFU and extended IFU were found to be 32% and 62%, respectively. This calls for active development and implementation of novel stent designs. FEVAR is especially useful in that it addresses the etiology of hostile aortic neck development, which is a progressive shortening of the neck. Although FEVAR is yet to be introduced as an available treatment option in Korea, it has already demonstrated reliable results in many studies [6711]. Our study offers an understanding of the anatomic characteristic of the Korean AAA population, which is crucial for the implementation of FEVAR.
Complex aortic neck is predominantly determined by the anatomical quality of the infrarenal neck, although the exact criteria that constitute neck complexity are controversial. Aortic neck characteristics outside the objective criteria of a stent graft’s IFU are considered a pragmatic definition of complex aortic neck used by the National Institute for Health and Care Excellence of the United Kingdom [12], as in this study. Up to 60% of AAAs are reported to be complex in the United States [13], whereas this study reported 51.2% of Korean AAA patients to have a complex aortic neck. Juxtarenal AAAs account for approximately 15% of all AAAs, and this complies with the result of this study where 18.5% of AAA patients exhibited such short aortic neck.
In this study, 33.3% of patients with juxtarenal AAAs were found to be feasible for the application of Z-FEN (13 out of 39 patients). This result mirrors that of a prior study which also deemed 33% of its patients feasible for Z-FEN [11]. Meanwhile, another study demonstrated that the p-Branch (Cook Medical, Brisbane, Australia), a FEVAR product of the same company which allows longer proximal sealing zones by placement of an additional fenestration, was applicable to 66% of patients with juxtarenal aortic aneurysms [14]. Together, it would be safe to believe that FEVAR has the potential to become a significant treatment option for juxtarenal AAAs in Korea.
The most common reason for unsuitability for Z-FEN was found to be severe angulation (53.8%), followed by short aortic neck length (38.5%) in this study. Previous research has also found severe neck angulation as the most common reason for unsuitability of conventional EVAR in the Korean population (39%) [10]. This similarity might be a reflection of intrinsic anatomic characteristics of the Korean population. It is known that common anatomic barriers for EVAR in western patients are short neck length and large neck diameter, rather than hostile angulation [2]. Indeed, a study conducted on American patients on the feasibility of Z-FEN reports inadequate proximal neck length as the greatest contributor to anatomic exclusion (46.9%), and exclusion due to severe angulation was reported to be 18.0% [7].
The same study also remarked that the most common device configuration, used in 60% of the patients, consisted of 2 small renal fenestrations and a scallop for the SMA. This finding was also observed in this study, where the design covered 61.5% of the eligible patients. In cases where such design was applicable, the most common clock positioning for the vessels was 12 o’clock for SMA, 9 o’clock for the right renal artery, and 3 o’clock for the left renal artery. In cases that required clock positions other than those, the deviation did not exceed 30 minutes in clock position. Therefore, we suggest that although Z-FEN was developed to allow for customization in accordance with anatomical variations, a standardized design of 2 renal fenestrations and an SMA scallop could prove useful in many cases, either as an off-the-shelf device or a stocked standard device. The authors expect it would help in overcoming several shortcomings of custom-made fenestrated devices such as 4 to 8 weeks of time delay for customization, complex implantation procedure, and high cost [15].
Apparently, custom-made devices are able to cope with anatomically challenging aortic neck, but not all patients can afford it or have the 2-month to 3-month time of delay for the customization. In contrast, premade devices including p-Branch, which was developed to cover AAAs originating below SMA and reinforce the sealing zone to avoid stent fracture and endoleak, are applicable in emergency cases [16]. For example, in ruptured AAA patients, only premade devices can be used to perform EVAR, and recent studies comparing emergency EVAR and open surgery repair reported that emergency EVAR tended to exhibit lower mortality [1718]. Both premade devices and custom-made devices are inapplicable in cases of severe neck angulation. The authors believe that premade devices with 1 scallop and 2 fenestrations can be a good option for patients with ruptured AAAs and those who cannot afford custom-made devices, though it would be best if both devices were available to complement each other if possible.
The other option for patients with hostile aortic necks is endoanchors. Endoanchors were developed to prevent type 1a endoleak and graft migration by penetrating both the stent-graft fabric and aortic wall with metallic “tack” resulting in a more secure proximal sealing zone. Recent systemic review regarding the use of endoanchors suggests that endoanchor fixation is a technically feasible and safe procedure with similar outcomes in comparison to the latest generation of stent grafts [19]. Though current evidence lacks long-term follow-up and routine application of endoanchors is not recommended [5], the authors believe it can be a helpful option when addressing a challenging aortic neck with insufficient proximal sealing zone. In cases where fenestrated stent graft is applicable and thereby enough sealing zone could be secured, endoanchor fixation can be considered as a secondary procedure.
Severe neck angulation was the most common reason for unsuitability in the non-feasible group. However, in the real world, surgeons perform EVAR on patients with anatomical characteristics outside the IFU if deemed feasible, and angles between 45° and 60° are one of those criteria that could be overcome. Considering this, of the 26 patients in the Z-FEN non-feasible group, Z-FEN could still be a clinical treatment option for 11 patients (42.3%).
This study has several limitations. The current recommendation for Z-FEN planning requires the CT slice thickness to be within 2 mm since the 3D reconstructed image cannot accurately represent the actual anatomy with thicker slices. However, the slice thickness for some of the CT images used in this study ranged from 3.75 to 5 mm. It is also noteworthy that since the study population was limited to a single institution, our AAA population may not be representative of the general patient population of Korea.
In conclusion, FEVAR is currently recommended over open repair in patients with juxtarenal AAAs according to the most recent practice guidelines published by the European Society for Vascular Surgery [5]. The feasibility, safety, and effectiveness of different FEVAR devices, including Z-FEN, have also been proven repeatedly by many prior studies. This study is meaningful in that it is the first to study the feasibility of FEVAR in Korean patients and, in doing so, provides the basis for developing FEVAR devices that suit the vascular anatomy of Korean juxtarenal AAA patients.
Go to : 
References
1. Lederle FA, Freischlag JA, Kyriakides TC, Matsumura JS, Padberg FT, Kohler TR, et al. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med. 2012; 367:1988–1997. PMID: 23171095.
2. Timaran CH, Rosero EB, Smith ST, Modrall JG, Valentine RJ, Clagett GP. Influence of age, aneurysm size, and patient fitness on suitability for endovascular aortic aneurysm repair. Ann Vasc Surg. 2008; 22:730–735. PMID: 18834703.
3. Verhoeven EL, Katsargyris A, Oikonomou K, Kouvelos G, Renner H, Ritter W. Fenestrated endovascular aortic aneurysm repair as a first line treatment option to treat short necked, juxtarenal, and suprarenal aneurysms. Eur J Vasc Endovasc Surg. 2016; 51:775–781. PMID: 26860255.
4. British Society for Endovascular Therapy and the Global Collaborators on Advanced Stent-Graft Techniques for Aneurysm Repair (GLOBALSTAR) Registry. Early results of fenestrated endovascular repair of juxtarenal aortic aneurysms in the United Kingdom. Circulation. 2012; 125:2707–2715. PMID: 22665884.
5. Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. Editor’s choice: European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019; 57:8–93. PMID: 30528142.
6. Oderich GS, Farber MA, Schneider D, Makaroun M, Sanchez LA, Schanzer A, et al. Final 5-year results of the United States Zenith Fenestrated prospective multicenter study for juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2021; 73:1128–1138.e2. PMID: 32891806.
7. Oderich GS, Greenberg RK, Farber M, Lyden S, Sanchez L, Fairman R, et al. Results of the United States multicenter prospective study evaluating the Zenith fenestrated endovascular graft for treatment of juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2014; 60:1420–1428.e1. PMID: 25195145.
8. Oderich GS, Forbes TL, Chaer R, Davies MG, Lindsay TF, Mastracci T, et al. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J Vasc Surg. 2021; 73:4S–52S. PMID: 32615285.
9. AbuRahma AF, Yacoub M, Mousa AY, Abu-Halimah S, Hass SM, Kazil J, et al. Aortic neck anatomic features and predictors of outcomes in endovascular repair of abdominal aortic aneurysms following vs not following instructions for use. J Am Coll Surg. 2016; 222:579–589. PMID: 26905372.
10. Hwang D, Kim J, Kim HK, Huh S. Suitability of the aortic neck anatomy for endovascular aneurysm repair in Korean patients with abdominal aortic aneurysm. Vasc Specialist Int. 2020; 36:71–81. PMID: 32611839.
11. Greenberg RK, Sternbergh WC, Makaroun M, Ohki T, Chuter T, Bharadwaj P, et al. Intermediate results of a United States multicenter trial of fenestrated endograft repair for juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2009; 50:730–737.e1. PMID: 19786236.
12. National Institute for Health Care Excellence (NICE). Abdominal aortic aneurysm: diagnosis and management. NICE guideline NG156 [Internet]. London: NICE;2020. cited 2020 Mar 19. Available from: https://www.nice.org.uk/guidance/ng156.
13. Aburahma AF, Campbell JE, Mousa AY, Hass SM, Stone PA, Jain A, et al. Clinical outcomes for hostile versus favorable aortic neck anatomy in endovascular aortic aneurysm repair using modular devices. J Vasc Surg. 2011; 54:13–21. PMID: 21324631.
14. Mendes BC, Oderich GS, Macedo TA, Pereira AA, Cha S, Duncan AA, et al. Anatomic feasibility of off-the-shelf fenestrated stent grafts to treat juxtarenal and pararenal abdominal aortic aneurysms. J Vasc Surg. 2014; 60:839–847. discussion 847-8. PMID: 24998837.
15. Haulon S, Barillà D, Tyrrell M, Tsilimparis N, Ricotta JJ. Debate: whether fenestrated endografts should be limited to a small number of specialized centers. J Vasc Surg. 2013; 57:875–882. PMID: 23446130.
16. Kitagawa A, Greenberg RK, Eagleton MJ, Mastracci TM. Zenith p-branch standard fenestrated endovascular graft for juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2013; 58:291–300. PMID: 23611709.
17. IMPROVE Trial Investigators. Comparative clinical effectiveness and cost effectiveness of endovascular strategy v open repair for ruptured abdominal aortic aneurysm: three year results of the IMPROVE randomised trial. BMJ. 2017; 359:j4859. PMID: 29138135.
18. Choo SJ, Jeon YB, Oh SS, Shinn SH. Outcomes of emergency endovascular versus open repair for abdominal aortic aneurysm rupture. Ann Surg Treat Res. 2021; 100:291–297. PMID: 34012947.
19. Qamhawi Z, Barge TF, Makris GC, Patel R, Wigham A, Anthony S, et al. Editor’s choice: systematic review of the use of endoanchors in endovascular aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2020; 59:748–756. PMID: 32192844.
Go to : 




 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download