Abstract
Purpose
In general, bile is normally sterile. However, there are reports bactibilia may occur in certain instances, though the causal factors are unclear. We analyzed possible preoperative predictors of bactibilia upon cholecystectomy.
Methods
Bile samples were collected during cholecystectomies from November 2018 to November 2019. A total of 428 open or laparoscopic cholecystectomies were performed. Preoperative, intraoperative, and postoperative variables were compared between the culture-positive and culture-negative groups.
Results
One hundred fifty-seven patients (36.7%) were culture-positive. Gram-negative bacteria (95 [61.0%]) were more common. Escherichia coli (38 [40.0%]) and Enterobacter (22 [23.2%]) were the most common species. In univariate analysis, age of ≥70 years (P < 0.001), male sex (P < 0.001), high American Society of Anesthesiologists physical status grades (P = 0.001), diabetes mellitus (P = 0.002), jaundice (P = 0.007), high Tokyo Guideline grades (P = 0.008), percutaneous transhepatic gallbladder drainage (PTGBD; P < 0.001), endoscopic retrograde cholangiopancreatography (ERCP; P < 0.001) were identified as a risk factors for bactibilia. In multivariate analysis, age of ≥70 years (hazard ratio [HR], 2.874; 95% confidence interval [CI], 1.769–4.670; P = 0.001), ERCP (HR, 9.001; 95% CI, 4.833–16.75; P < 0.001), and PTGBD (HR, 2.866; 95% CI, 1.440–4.901; P = 0.002) were independent risk factors for bactibilia.
Go to : 
Bile is a complex aqueous secretion that originates from hepatocytes [1]. It is modified by absorptive and secretory transport systems in the bile duct epithelium and eventually enters the gallbladder (GB), where it is concentrated or delivered directly to the intestinal lumen [2]. After entering the intestinal lumen, bile is excreted in feces or returned to the liver through the portal vein [3]. Throughout this process, bile is typically sterile [4].
However, bacteria can start to grow in bile under several conditions, and this is called bactibilia [56]. Bactibilia can cause severe inflammatory conditions such as cholecystitis, cholangitis, and sepsis. Therefore, it is very important to determine the epidemiology of bactibilia and to elucidate risk factors related to its occurrence, in order to predict and improve the clinical outcomes of patients [5]. Several factors, including old age, previous endoscopic retrograde cholangiopancreatography (ERCP), and acute cholecystitis, have been identified as risk factors for bactibilia [478]. However, microbiology may differ between areas and hospitals (i.e., community hospital vs. tertiary referral hospital); therefore, it is necessary to investigate this issue at different regions or institutions [91011]. The present study aimed to identify the causative microorganisms and preoperative risk factors for bactibilia in a community hospital in South Korea.
Go to : 
This was a retrospective cohort study using prospectively collected medical data. Between November 2018 and November 2019, 463 patients underwent open or laparoscopic cholecystectomies for benign GB disease at our institution. Among them, 35 patients were excluded because they underwent other operations in addition to cholecystectomy or did not consent to be included in this study. Thus, 428 patients were included in this study. Two experienced hepatobiliary surgeons performed cholecystectomy throughout the study period. This study was approved by the Institutional Review Board of Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea, and all patients provided written informed consent to participate in this study (No. 20-2018-79).
Patients’ medical data were prospectively collected. Basic clinical variables, such as sex, age, American Society of Anesthesiologists physical status (ASA PS) classification, and chief complaints (e.g., abdominal pain, fever, and jaundice), were investigated. Preoperative radiologic findings were also investigated to identify GB stones, common bile duct (CBD) stones, CBD dilatation, and cholangiohepatitis. The patients who were suspicious of acute cholecystitis were classified according to the diagnostic and severity grading criteria of the Tokyo Guidelines [12]. In addition, the performance of preoperative procedures was analyzed, and bile culture results during cholecystectomy were collected. All patients underwent preoperative CT using Elite 128 (Philips Medical Systems, Cleveland, OH, USA), Ingenuity 128 (Philips Medical Systems), or Revolution (GE, Windsor, CT, USA).
A second-generation cephalosporin antibiotic was intravenously administered prior to elective surgery, while a 3rd-generation cephalosporin antibiotic and metronidazole were administered in emergency surgery. When the patient’s vital signs were unstable due to severe cholecystitis, or preoperative evaluation was necessary due to underlying comorbidities, percutaneous transhepatic GB drainage (PTGBD) was inserted, and elective surgery was performed after the patient’s condition had stabilized and preoperative evaluation had been completed. Additionally, ERCP was performed when the CBD was occluded by stones, and endoscopic retrograde biliary drainage was also inserted simultaneously.
Bile samples were collected aseptically at the time of surgery by swabs. For laparoscopic cholecystectomy, the first step was ligation of the cystic artery and cystic duct. Then, the GB was carefully separated from the liver, placed in a LapBag (Sejong Medical Co., Paju, Korea), and removed from the umbilicus. The swab was taken by creating a hole in the GB inside the bag before removing it through the umbilicus. In cases of open cholecystectomy, the GB was punctured upon its removal, and a swab was taken. Cotton swabs (COPAN, Brescia, Italy) were carefully evaluated for aerobic, anaerobic, and fungal organisms by routine cultivation for 48 hours at 35℃ in 5% carbon dioxide in blood agar plate and MacConkey agar.
The chi-square test and Fisher exact test were used to evaluate qualitative data. The Student t-test and Mann-Whitney U-test were used to quantitatively compare the 2 groups. Logistic regression was used for multivariate analysis. All statistical analyses were performed using IBM SPSS Statistics ver. 25.0 (IBM Corp., Armonk, NY, USA). Statistical significance was set at P < 0.05.
Go to : 
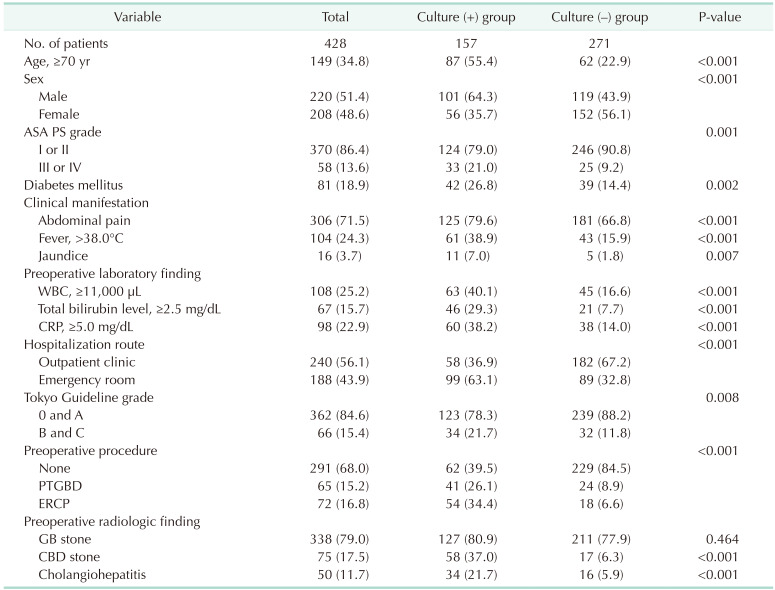
Detailed clinicopathologic findings of the patients are described in Table 1. In total, 157 patients (36.7%) had positive bile culture results. The mean age of patients was 59.2 ± 16.5 years (range, 20–96 years). In total, 306 patients (71.5%) had abdominal pain, 104 (24.3%) had a preoperative fever higher than 38℃, and 16 (3.7%) had jaundice. Fifty-eight patients (13.6%) had an ASA PS grade of III or IV. One hundred thirty-seven patients (32.0%) underwent preoperative procedures, 72 (16.8%) underwent ERCP, and 65 (15.2%) underwent PTGBD.
Culture-positive patients were older (≥70 years, P < 0.001) and had a higher proportion of males (101 [64.3%] vs. 56 [35.7%], P < 0.001) than culture-negative patients. Culture-positive patients also had preoperative fever (>38.0℃) significantly more frequently than culture-negative patients (61 [38.9%] vs. 43 [15.9%], P < 0.001). The rate of preoperative drainage insertion was higher in culture-positive patients than in culture-negative patients. Among preoperative radiologic variables, the percentages of patients with CBD stones (58 [37.0%] vs. 17 [6.3%], P < 0.001) and cholangiohepatitis (34 [21.7%] vs. 16 [5.9%], P < 0.001) were significantly higher in the culture-positive group than in the culture-negative group. The grades of cholecystitis according to Tokyo Guidelines were higher in the culture-positive group (P = 0.008) [12]. Several preoperative laboratory findings differed significantly between the 2 groups. The percentages of patients with leukocytosis (WBC count of ≥11,000 µL; 63 [40.1%] vs. 45 [16.6%], P < 0.001), total bilirubin level of ≥2.5 mg/dL (46 [29.3%] vs. 21 [7.7%], P < 0.001), and CRP level of ≥5.0 mg/dL (60 [38.2%] vs. 38 [14.0%], P < 0.001) were significantly higher in the culture-positive group than in the culture-negative group (Table 2).
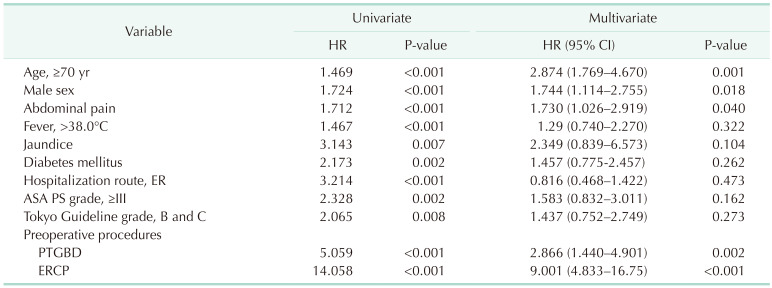
In multivariate analysis, age of ≥70 years (hazard ratio [HR], 2.874; 95% confidence interval [CI], 1.769–4.670; P = 0.001), male sex (HR, 1.744; 95% CI, 1.114–2.755; P = 0.018), abdominal pain (HR, 1.730; 95% CI, 1.026–2.919; P = 0.040), PTGBD (HR, 2.866; 95% CI, 1.440–4.901; P = 0.002), and ERCP (HR, 9.001; 95% CI, 4.833–16.75; P < 0.001) were independent risk factors for bactibilia (Table 3).
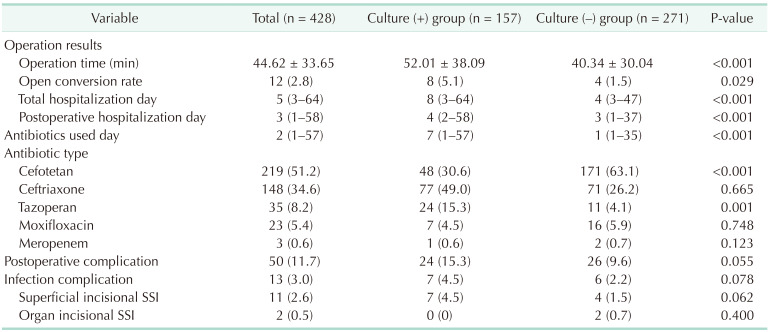
Perioperative factors were also investigated. The most commonly used antibiotic was cefotetan (219 [51.2%]), followed by ceftriaxone with metronidazole (97 [22.7%]), ceftriaxone (51 [11.9%]), piperacillin-tazobactam (35 [8.2%]), moxifloxacin (23 [5.4%]), and meropenem (3 [0.7%]). Overall, the duration of antibiotic use was longer in the group that underwent preoperative ERCP or PTGBD than in that of no procedure (median of 8 days vs . 1 day, P = 0.002). Culture-positive patients had a longer operation time (52.0 ± 38.1 minutes vs. 40.3 ± 30.0 minutes, P < 0.001), a higher open conversion rate (8 [5.1%] vs. 4 [1.5%], P = 0.029], longer overall hospitalization (12.08 ± 10.97 days vs. 7.26 ± 6.63 days, P < 0.001), a longer postoperative hospital stay (7.11 ± 8.52 days vs. 4.58 ± 4.77 days, P < 0.001), and longer antibiotic administration (9.04 ± 8.98 days vs. 4.04 ± 5.63 days, P < 0.001) than culture-negative patients. However, the percentages of patients with postoperative complications (24 [15.3%] vs. 26 [9.6%], P = 0.055) including overall infectious complications (7 [4.5%] vs. 6 [2.2%], P = 0.078) and surgical site infection (7 [4.5%] vs. 4 [1.5%], P = 0.062) did not significantly differ between the 2 groups.
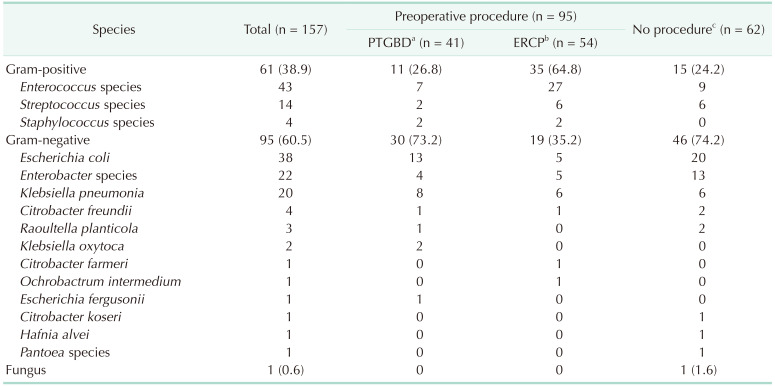
Among the 157 positive culture tests, gram-positive bacteria were detected in 61 patients (38.9%), and gram-negative bacteria were detected in 95 (60.5%). Among gram-positive bacteria, Enterococcus species was the most common (43 [70.5%]), followed by Streptococcus species (14 [23.0%]) and Staphylococcus species (4 [6.6%]). Among gram-negative bacteria, Escherichia coli was the most common (38 [40.0%]), followed by Enterobacter species (22 [23.2%]). Klebsiella pneumonia, Citrobacter freundii, Raoultella planticola, Klebsiella oxytoca, Citrobacter farmeri, Ochrobactrum intermedium, Escherichia fergusonii, Citrobacter koseri, Hafnia alvei, and Pantoea species were also identified (Table 4).
Among 137 patients who underwent preoperative procedures, 95 patients (69.3%) had positive culture results (Table 4). Among these patients, gram-positive bacteria were detected in 46 (48.4%), and gram-negative bacteria were detected in 49 (51.6%). Among patients who did not undergo preoperative procedures, gram-negative bacteria were more commonly identified than gram-positive bacteria (46 [74.2%] vs. 15 [24.2%]). Gram-positive bacteria were more commonly identified in patients who underwent ERCP than in patients who underwent PTGBD and patients who did not undergo preoperative procedures (35 [64.8%] vs. 11 [26.8%], P < 0.001 and 35 [64.8%] vs. 15 [24.2%], P < 0.001, respectively). However, gram-negative bacteria were more commonly identified in patients who underwent PTGBD, but there was no significant difference from patients who did not undergo preoperative procedures (30 [73.2%] vs. 46 [74.2%], P = 0.799).
Go to : 
The effects of bactibilia on the prognosis of patients have been extensive. However, since most of the studies were conducted in a tertiary hospital, their results were based on patients who underwent primary treatment at primary and secondary institutions [9101113]. Our institution, where the study was conducted, is a community hospital that requires primary treatment for the patients who came through emergency room or outpatient clinic. Hence, the treatment policy should be different depending on the presence of bacteria before surgery. Therefore, the significance of this paper is to identify preoperative risk factors that can determine the presence of bactibilia, and to identify the types of bacteria frequently present in the community for more appropriate treatment.
In our study, we found a relationship between old age and bactibilia. We found that an age of ≥70 years was a risk factor for bactibilia (HR, 2.874; P = 0.001) and it was consistent with another previous study [14]. Based on several reports that human immunity decreases with aging, which increases susceptibility to infection, old age can be considered a risk factor for bactibilia [1516]. Male sex was also a risk factor for bactibilia in our study (HR, 1.744; P = 0.018). Maseda et al. [17] reported that male sex is a significant risk factor for bactibilia. Conversely, Galili et al. [5] and other investigators [7], concluded that sex is not a significant factor for culture outcomes. Given these conflicting results, a study with more patients is required to obtain a more reliable conclusion.
In our study, among patients who underwent preoperative PTGBD, 2/3 had positive culture results, and gram-negative bacteria were detected in 3-quarters of these patients, which is in line with previous studies [1819]. The possibility of an ascending infection due to PTGBD cannot be excluded. However, given that the bacteria differs from normal skin flora, and is similar to the bacteria from no-procedure group, it is reasonable to assume that the PTGBD procedure itself would not have caused bactibilia (Table 4). Moreover, the patients who underwent a preoperative PTGBD procedure were more likely to have severe cholecystitis, which makes them unable to receive upfront cholecystectomy. It is presumed that such a comprehensive situation of patients requiring preoperative PTGBD insertion is related to bactibilia.
In our study, preoperative ERCP was a major risk factor for bactibilia. Gram-positive bacteria accounted for about 2/3 of positive results, and Enterococcus species were most commonly identified. Sghir et al. [20] reported that Enterococcus species are detected in normal bowel contents of humans, and several studies have demonstrated that preoperative ERCP is a risk factor for bactibilia [82122]. Considering that the bacterial distribution of patients who underwent ERCP differed from that of patients who did not undergo preoperative procedures in the present study, bactibilia may have resulted from ascending infection of the bowels (Table 4). Many studies have suggested that an ascending infection of the bile duct starting from the duodenum due to sphincterotomy can be responsible for positive culture results after preoperative ERCP [821]. When sphincterotomy is performed, the pressure of the bile duct decreases, resulting in a retrograde flow of the bowel’s contents [23]. In addition, Ruan et al. [24] suggested that ERCP destroys the normal anatomy of the bile tract and increases bile reflux due to biliary tract motor dysfunction. As bile reflux increases, inflammatory edema may occur in the bile mucosa, which ultimately creates an environment favorable for bacterial growth and colonization.
In contrast to the present study, there have been several studies where gram-negative bacteria were identified more frequently than gram-positive bacteria for the patients who underwent ERCP [2526]. However, among gram-negative bacteria, the most commonly identified microorganisms varied from institution to institution. Manrai et al. [22] have reported that E. coli was the most common and Bass et al. [27] reported that Pseudomonas was the most common gram-negative bacteria. Du et al. [28] reported that Enterococcus was the most commonly identified bacteria in the patients who underwent ERCP, which is in line with the present study. Furthermore, bacteria occurred in 41 of 65 patients who underwent PTGBD (Table 4), and 73% of patients were gram-negative bacteria, so it would be helpful to the patient to use antibiotics that cover at least gram-negative bacteria before surgery. In essence, these different culture results might have resulted from differences in the distribution of bacteria according to regions or hospitals. However, the exact reasons have not been clarified yet and further large-scale studies from various institutions in different regions are required.
Various bacteria, including gram-positive and gram-negative bacteria, and even a fungus, were identified (gram-positive, 61 [38.9%] and gram-negative, 95 [60.5%]). Many other studies described a higher portion of gram-negative bacteria (66.2%–75.9%) [192930]. However, in the study by Rupp et al. [31], which was conducted at a tertiary hospital in Germany, gram-positive bacteria were identified in 840 patients (57%), and gram-negative bacteria were identified in 651 patients (43%). These differences in microorganisms present in bile might be due to regional or institutional differences. Therefore, clinicians should understand these variations in terms of epidemiology to choose the optimal antibiotics and improve the clinical outcomes of patients [910].
The present study showed that the operation time, open conversion rate, length of hospitalization, and length of antibiotic administration were higher among culture-positive patients than among culture-negative patients. Although the relationship between culture-positive and complication was not significantly found in our paper (P = 0.055), as asserted by several other papers, bactibilia increases the incidence of postoperative complications [3233]. Therefore, culture-positive patients should be more appropriately and aggressively controlled for infection than culture-negative patients.
This study has several limitations. First, there is a possibility of selection bias because the study was conducted at a single institution. However, this study provides information on the incidence of bactibilia and types of causative bacteria to clinicians working in community hospitals, where patients with gallstone-related symptoms visit for the first time, so that clinicians can provide appropriate treatment. Second, it is possible that the frequency and categories of administered intravenous antibiotics differed between elective and emergency surgeries, which may have affected the bile culture results. However, in patients who did not undergo preoperative procedures, the average number of days of antibiotic use before surgery was 0.85 days, which may have been insufficient to reach the therapeutic dose of antibiotics. Third, the period of antibiotics was longer in the procedure group than in the no-procedure group (median of 8 days vs. 1 day, P = 0.002), and the ratio of the culture-positive patient was higher in the procedure group. Lastly, there is a possibility of contamination during the PTGBD insertion, such as ascending infection of skin normal flora through the tube. However, the composition of bacteria in patients between PTGBD and no procedure was comparable (Table 4). Therefore, this study demonstrates that patients who undergo procedures prior to cholecystectomy have a high possibility of having bactibilia, and caution should be taken.
In conclusion, bactibilia occurred in about 1/3 of patients, and patients of male sex, older than 70 years, with abdominal symptom, and who underwent preoperative PTGBD or ERCP, were likely to have bactibilia. Surgeons should keep in mind that bactibilia might exist in those patients and should take care during operation to avoid spillage of infected bile into the peritoneal cavity [34]. Since the causative bacteria differ depending on the case of PTGBD or ERCP, it is necessary to use an antibiotic suitable for each case.
Go to : 
Notes
Fund/Grant Support: This work was supported by a general clinical research grant-in-aid from the Seoul Metropolitan Government-Seoul National University (SMG-SNU) Boramae Medical Center (03-2018-26).
Go to : 
References
1. Hundt M, Basit H, John S. Physiology, bile secretion. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing;2022. updated 2022 Sep 26. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470209/
.
2. Boyer JL. Bile formation and secretion. Compr Physiol. 2013; 3:1035–1078. PMID: 23897680.
3. Cai JS, Chen JH. The mechanism of enterohepatic circulation in the formation of gallstone disease. J Membr Biol. 2014; 247:1067–1082. PMID: 25107305.
4. Morris-Stiff GJ, O’Donohue P, Ogunbiyi S, Sheridan WG. Microbiological assessment of bile during cholecystectomy: is all bile infected? HPB (Oxford). 2007; 9:225–228. PMID: 18333227.
5. Galili O, Eldar S, Matter I, Madi H, Brodsky A, Galis I, et al. The effect of bactibilia on the course and outcome of laparoscopic cholecystectomy. Eur J Clin Microbiol Infect Dis. 2008; 27:797–803. PMID: 18369670.
6. Thompson JE, Bennion RS, Doty JE, Muller EL, Pitt HA. Predictive factors for bactibilia in acute cholecystitis. Arch Surg. 1990; 125:261–264. PMID: 2302066.
7. Darkahi B, Sandblom G, Liljeholm H, Videhult P, Melhus Å, Rasmussen IC. Biliary microflora in patients undergoing cholecystectomy. Surg Infect (Larchmt). 2014; 15:262–265. PMID: 24801654.
8. Yun SP, Seo HI. Clinical aspects of bile culture in patients undergoing laparoscopic cholecystectomy. Medicine (Baltimore). 2018; 97:e11234. PMID: 29952986.
9. Kuo CC, Lai CC, Chao CM. Bile microbiology at a hospital in southern Taiwan. Infection. 2013; 41:901–902. PMID: 23381877.
10. Kwon W, Jang JY, Kim EC, Park JW, Han IW, Kang MJ, et al. Changing trend in bile microbiology and antibiotic susceptibilities: over 12 years of experience. Infection. 2013; 41:93–102. PMID: 23180506.
11. Kwon W, Kim SW, Jang JY. Differences in bile microbiology according to region and hospital: response to correspondence on “Bile microbiology at a hospital in southern Taiwan”. Infection. 2013; 41:1037–1038. PMID: 23397257.
12. Yokoe M, Hata J, Takada T, Strasberg SM, Asbun HJ, Wakabayashi G, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018; 25:41–54. PMID: 29032636.
13. Jain N, Neogi S, Bali RS, Harsh N. Relationship of gallbladder perforation and bacteriobilia with occurrence of surgical site infections following laparoscopic cholecystectomy. Minim Invasive Surg. 2015; 2015:204508. PMID: 26605081.
14. Oliveira RS, Silva PD, Queiroz CA, Terra-Júnior JA, Crema E. Prevalence of bacteriobilia in patients undergoing elective colecystectomy. Arq Bras Cir Dig. 2018; 31:e1392. PMID: 30133684.
15. Sadighi Akha AA. Aging and the immune system: an overview. J Immunol Methods. 2018; 463:21–26. PMID: 30114401.
16. Müller L, Di Benedetto S, Pawelec G. The immune system and its dysregulation with aging. Subcell Biochem. 2019; 91:21–43. PMID: 30888648.
17. Maseda E, Maggi G, Gomez-Gil R, Ruiz G, Madero R, Garcia-Perea A, et al. Prevalence of and risk factors for biliary carriage of bacteria showing worrisome and unexpected resistance traits. J Clin Microbiol. 2013; 51:518–521. PMID: 23196362.
18. Nitzan O, Brodsky Y, Edelstein H, Hershko D, Saliba W, Keness Y, et al. Microbiologic data in acute cholecystitis: ten years’ experience from bile cultures obtained during percutaneous cholecystostomy. Surg Infect (Larchmt). 2017; 18:345–349. PMID: 28394748.
19. Yoon JH, Paik KY, Chung HY, Oh JS. Clinical implication of bactibilia in moderate to severe acute cholecystitis undergone cholecystostomy following cholecystectomy. Sci Rep. 2021; 11:11864. PMID: 34088947.
20. Sghir A, Gramet G, Suau A, Rochet V, Pochart P, Dore J. Quantification of bacterial groups within human fecal flora by oligonucleotide probe hybridization. Appl Environ Microbiol. 2000; 66:2263–2266. PMID: 10788414.
21. Mahafzah AM, Daradkeh SS. Profile and predictors of bile infection in patients undergoing laparoscopic cholecystectomy. Saudi Med J. 2009; 30:1044–1048. PMID: 19668885.
22. Manrai M, Jha AA, Singh Shergill SP, Thareja S, Sood AK, Shukla R, et al. Microbiology of bile in extrahepatic biliary obstruction: a tropical experience. Indian J Med Microbiol. 2021; 39:54–58. PMID: 33610257.
23. Chen M, Wang L, Wang Y, Wei W, Yao YL, Ling TS, et al. Risk factor analysis of post-ERCP cholangitis: a single-center experience. Hepatobiliary Pancreat Dis Int. 2018; 17:55–58. PMID: 29428105.
24. Ruan HQ, Liao GL, Peng P, Liu SQ, Wu CL, Qin JF, et al. Microbial profiles and risk factors of preexisting biliary infection in patients with therapeutic endoscopy. Gastroenterol Res Pract. 2019; 2019:1527328. PMID: 31191641.
25. Gargouri D, Ouakaa-Kchaou A, Kochlef A, Elloumi H, Bibani N, Trad D, et al. Microbiological study and antimicrobial susceptibility of bile in biliary therapeutic endoscopy. Tunis Med. 2015; 93:602–605. PMID: 26895121.
26. Kaya M, Beştaş R, Bacalan F, Bacaksız F, Arslan EG, Kaplan MA. Microbial profile and antibiotic sensitivity pattern in bile cultures from endoscopic retrograde cholangiography patients. World J Gastroenterol. 2012; 18:3585–3589. PMID: 22826624.
27. Bass DH, Oliver S, Bornman PC. Pseudomonas septicaemia after endoscopic retrograde cholangiopancreatography: an unresolved problem. S Afr Med J. 1990; 77:509–511. PMID: 2188380.
28. Du M, Suo J, Liu B, Xing Y, Chen L, Liu Y. Post-ERCP infection and its epidemiological and clinical characteristics in a large Chinese tertiary hospital: a 4-year surveillance study. Antimicrob Resist Infect Control. 2017; 6:131. PMID: 29299305.
29. Hadi YB, Waqas M, Umer HM, Alam A, Alvi AR, Khan MR, et al. Bacterobilia in acute cholecystitis: bile cultures’ isolates, antibiotic sensitivities and antibiotic usage. A study on a Pakistani population. J Pak Med Assoc. 2016; 66(Suppl 3):S50–S52.
30. den Hoed PT, Boelhouwer RU, Veen HF, Hop WC, Bruining HA. Infections and bacteriological data after laparoscopic and open gallbladder surgery. J Hosp Infect. 1998; 39:27–37. PMID: 9617682.
31. Rupp C, Bode K, Weiss KH, Rudolph G, Bergemann J, Kloeters-Plachky P, et al. Microbiological assessment of bile and corresponding antibiotic treatment: a strobe-compliant observational study of 1401 endoscopic retrograde cholangiographies. Medicine (Baltimore). 2016; 95:e2390. PMID: 26962768.
32. Velázquez-Mendoza JD, Alvarez-Mora M, Velázquez-Morales CA, Anaya-Prado R. Bactibilia and surgical site infection after open cholecystectomy. Cir Cir. 2010; 78:239–243. PMID: 20642907.
33. Armiñanzas C, Tigera T, Ferrer D, Calvo J, Herrera LA, Pajarón M, et al. [Role of bacteriobilia in postoperative complications]. Rev Esp Quimioter. 2016; 29:123–129. Spanish. PMID: 27062981.
34. Lee JM, Kim BW, Kim WH, Wang HJ, Kim MW. Clinical implication of bile spillage in patients undergoing laparoscopic cholecystectomy for gallbladder cancer. Am Surg. 2011; 77:697–701. PMID: 21679636.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download