Abstract
Purpose
Clinically, breast cancer can be divided into 4 subtypes based on the presence of hormone receptors, human epidermal growth factor receptor 2 (HER2), and Ki-67. Because the pattern and time of recurrence vary according to the subtype, we evaluated whether there was a difference in overall survival (OS) among the subtypes according to the time and type of recurrence.
Methods
A total of 2,730 patients who underwent breast cancer surgery were analyzed. Early and late recurrence were defined as recurrence within and after 5 years of diagnosis, respectively. Recurrence type was categorized as locoregional recurrence or systemic recurrence.
Results
Hormone receptor-positive tumors were significantly more frequent in the late recurrence group than in the early recurrence group (estrogen receptor positive, 47.8% [early] vs. 78.7% [late]). However, there was no difference in the rate of HER2 overexpression (HER2+, 38.1% [early] vs.39.0% [late]). In subgroup analysis, early recurrence was a significant prognostic factor for OS in all subtypes. However, late recurrence was a significant prognostic factor for OS only in the luminal B subtype (hazard ratio of 4.30). In addition, the luminal B type had the highest proportion in late recurrence patients (63.2%).
Go to : 
Breast cancer is a heterogeneous neoplasm. There are 4 subtypes according to gene expression profiling: luminal A, represented by hormone receptor-positive tumors with low proliferative activity; luminal B, also primarily represented by hormone receptor-positive tumors but with high proliferative activity; basal-like, which are mostly triple-negative breast cancers (TNBC) (estrogen receptor [ER]-negative, progesterone receptor [PR]-negative, and human epidermal growth factor receptor 2 [HER2]-negative); and HER2-positive, comprising tumors with high expression of the ERBB2 gene. These distinct molecular subtypes have differences in clinical features and prognosis [123].
The pattern and risk of recurrence vary according to subtype. Luminal A breast cancer has a low recurrence rate, but the risk of recurrence tends to increase slowly and steadily [4]. Luminal B breast cancer recurs at any time, both luminal A and B show persistent recurrence patterns, and luminal B shows a higher hazard ratio (HR) over time [3]. On the other hand, hormone receptor-negative breast cancer has a high rate of early recurrence, usually within 3 years, and subsequent recurrence is rare. HER2-positive breast cancer has a recurrence peak around 20 months. TNBC with a low Ki-67 level has a smooth risk curve, while TNBC with a high Ki-67 level shows a sharp peak near 18 months. TNBCs have an aggressive clinical course, but its effects are short. After 3 years, the recurrence peak is sharply reduced [5].
Recurrence type varies depending on the subtype. Hormone receptor-negative tumors have a higher risk of locoregional recurrence (LRR) than luminal breast cancer, and HER2-positive tumors have a higher risk of LRR and distant metastasis than HER2-negative tumors [6]. Patients with regional recurrence have a worse prognosis and higher risk of distant metastasis than patients with local recurrence [78].
Considering the different recurrence patterns according to subtype, we sought to determine whether there is a difference in prognosis according to the time of recurrence. In other words, we investigated whether there are differences in overall survival (OS) rate in patients with early recurrence and late recurrence depending on subtype.
Go to : 
This study was approved by the Institutional Review Board of Korea University Anam Hospital (No. 2019AN0126). It was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature.
A total of 2,730 patients who underwent surgery for breast cancer from January 2000 to May 2018 were enrolled. Women who were 20 years or older were included, and patients with distant metastasis at diagnosis were excluded.
We reviewed clinicopathological characteristics of patients, including biological factors such as the expression of ER, PR, HER2 and Ki-67. Pathological tumor stage was assessed according to the anatomical staging system of American Joint Committee on Cancer Staging Manual, 8th edition. When less than 1% of cells exhibit nuclear staining for ER and/or PR on immunohistochemistry (IHC), these cancers are defined as ‘ER-negative’ and ‘PR-negative’. A ‘HER2-negative’ cancer is defined as 0, +1 or +2 staining on IHC or in cases with a HER2/centromere of chromosome 17 copies ratio <2.0 on fluorescence in situ hybridization or silver in situ hybridization.
The patients were classified into 4 molecular subtypes as follows: luminal A (ER-positive [ER+] and/or PR-positive [PR+], HER2-negative [HER2–], and low Ki-67 level of <14.0%); luminal B (ER+ and/or PR+, Ki-67 of ≥14.0% or HER2-positve [HER2+]); HER2-positive (ER-negative [ER–], PR-negative [PR–], and HER2+); and triple-negative (ER–, PR–, and HER2–) breast cancer.
Recurrence was categorized as LRR or systemic recurrence. LRR was defined as local (in the ipsilateral breast, chest wall or skin) or regional (in the axillary lymph nodes or the supraclavicular region or in the internal mammary, supraclavicular, or infraclavicular nodes). Systemic recurrence was defined as distant metastasis that was detected after primary surgery. Contralateral breast recurrence was excluded because it could be a possible statistical bias. Early recurrence was defined as recurrence within 5 years of diagnosis. Late recurrence was defined as recurrence after 5 years from diagnosis.
The chi-square test was used to analyze differences between proportions. OS was assessed from the date of diagnosis to the date of death or last follow-up visit. OS was estimated using the Kaplan-Meier method and compared using the log-rank test. Independent prognostic factors for OS were identified by Cox multivariate proportional hazard analysis. All significant parameters on univariate analysis were included in a multivariate model. A P-value of <0.05 was considered statistically significant. IBM SPSS Statistics ver. 20.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Go to : 
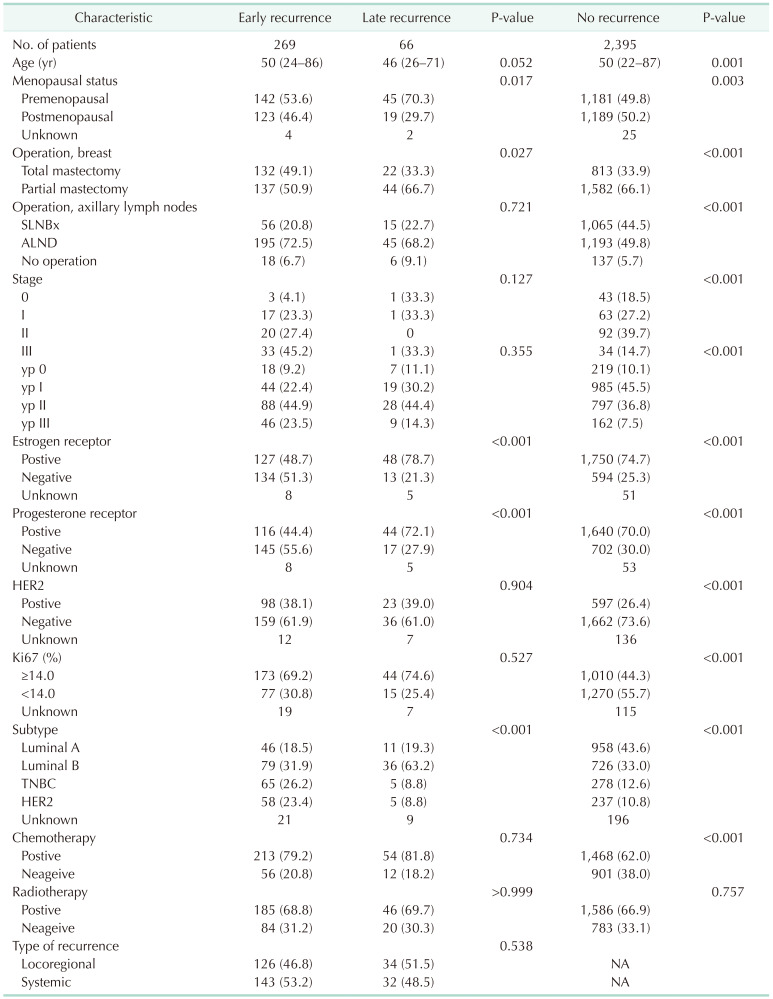
During the follow-up period in this study (median of 60 months; range, 1–289 months), recurrence occurred in 335 patients (12.3%). Of them, early recurrence was noted in 269 patients (80.3%), and late recurrence occurred in 66 cases (19.7%). The clinicopathologic characteristics of patients according to the time of recurrence are shown in Table 1. The median age of patients with late recurrence was 46 years, and they were younger (P = 0.052) and more likely to be premenopausal than patients with early recurrence (70.3% vs. 53.6%, P = 0.017). The rate of breast conserving surgery in the late recurrence group was higher (66.7% vs. 50.9%, P = 0.027), but there was no difference in stage between the 2 groups. The proportion of patients with hormone receptor-positive tumors was significantly higher in the late recurrence group (early vs. late: ER+, 48.7% vs. 78.7%; PR+, 44.4% vs. 72.1%; P < 0.001) than in the early recurrence group. However, there was no difference in the rate of HER2 overexpression (HER2+, 38.1% vs. 39.0%; P = 0.904). There was no difference in the proportion of patients who underwent chemotherapy or radiotherapy between the 2 groups.
Of the 335 patients who experienced recurrence, 160 patients (47.8%) had LRR and 175 patients (52.2%) had systemic recurrence (distant metastasis) as their first recurrence. For 34 of the patients with LRR (21.3%) and 32 of the patients with systemic recurrence (18.3%), the recurrence occurred after 5 years (i.e., late).
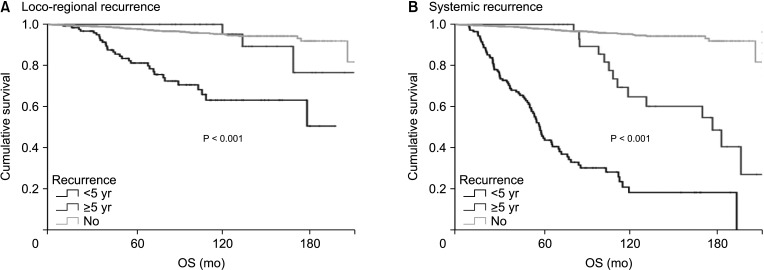
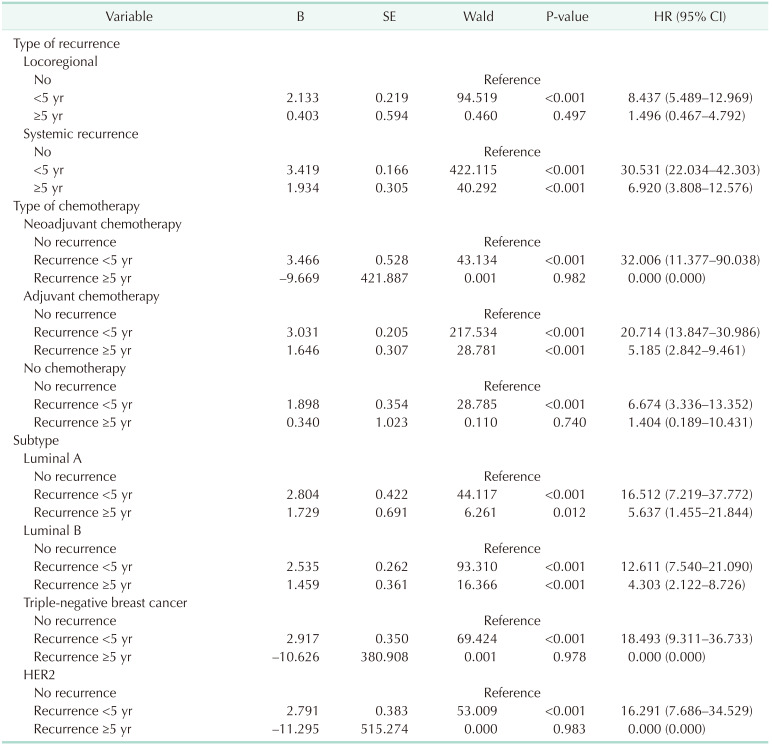
In patients with systemic recurrence, both early recurrence (HR, 30.5; 95% confidence interval [CI], 22.0–42.3) and late recurrence (HR, 6.9; 95% CI, 3.8–12.5) were significant prognostic factors for OS. However, in patients with LRR, early recurrence was a statistically significant prognostic factor (HR, 8.4; 95% CI, 5.4–12.9) for OS, but late recurrence was not (HR, 1.5; 95% CI, 0.5–4.8) (Fig. 1, Table 2).
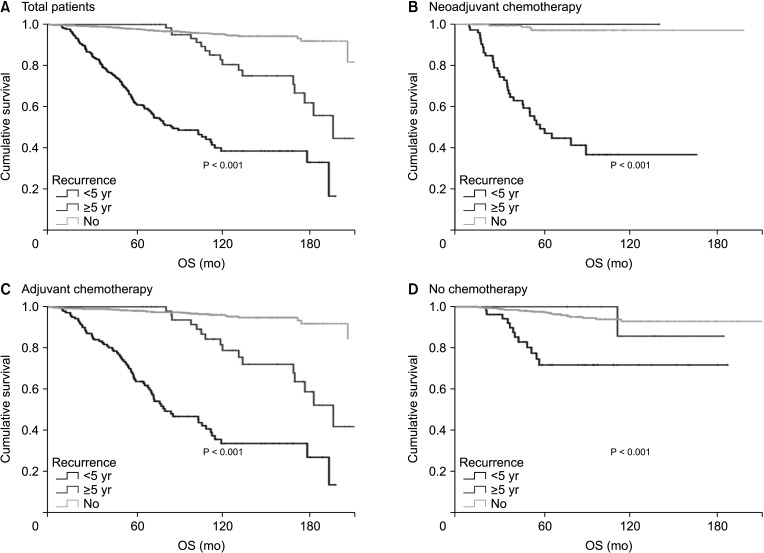
Of the 969 patients who did not receive chemotherapy, 68 (7.0%) had recurrences. Of these, 56 patients (82.4%) had recurrence within 5 years and 12 (17.6%) had recurrence after 5 years.
Of the 1,463 patients who underwent adjuvant chemotherapy, 192 patients (13.1%) had recurrence. Of these patients, 141 patients (73.4%) had recurrence within 5 years and 51 (26.6%) had recurrence after 5 years. Of the 308 patients who underwent neoadjuvant chemotherapy, 76 patients (24.7%) had recurrence. Of these, 72 patients (94.7%) had recurrence within 5 years and only 3 patients (4.2%) had recurrence after 5 years.
Patients with early recurrence showed a poor prognosis in all kinds of chemotherapy groups (no chemotherapy, adjuvant, and neoadjuvant chemotherapy) (Fig. 2). However, late recurrence was a significant prognostic factor for OS (HR, 5.18; 95% CI, 2.84–9.46 in the adjuvant chemotherapy only group). Late recurrence was not a significant prognostic factor for OS in patients who did not receive chemotherapy or in those who underwent neoadjuvant chemotherapy.
Of the 248 patients with recurrence within 5 years, 46 patients (18.5%) had luminal A, 79 (31.9%) had luminal B, 65 (26.2%) had TNBC, and 58 patients (23.4%) had HER2 disease. Of the 57 patients with recurrence after 5 years, 36 patients (63.2%) had luminal B, which was less than half of patients with this subtype who had early recurrence. About 92% each of recurrences of the TNBC and HER2 subtypes occurred within 5 years, and only 7.1% and 7.9%, respectively, of recurrences occurred after 5 years.
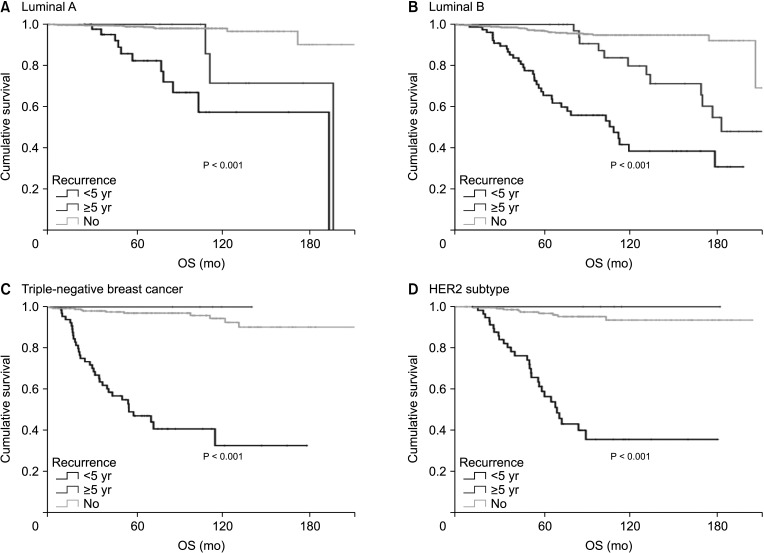
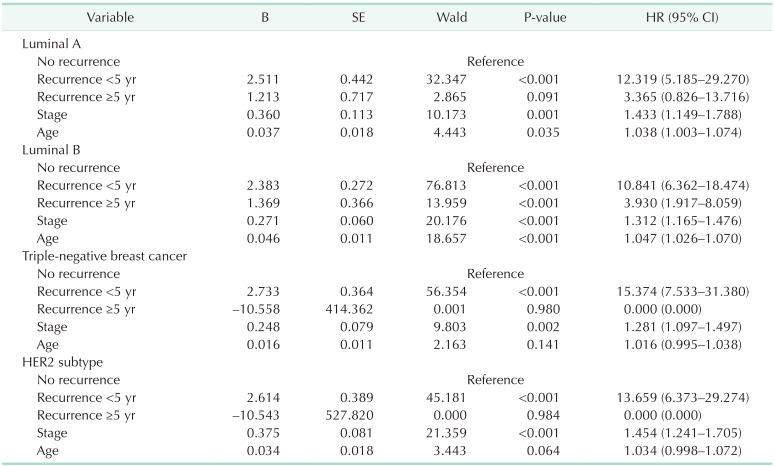
Subgroup analysis by subtype showed that early recurrence was a significant prognostic factor for OS in all subtypes (Fig. 3, Table 3). However, late recurrence was a significant prognostic factor for OS with a HR of 3.93 (95% CI, 1.917–8.059) in the luminal B subtype only.
Go to : 
Early recurrence was a prognostic factor for OS in patients with LRR, late recurrence was not a significant prognostic factor. However, in patients with systemic recurrence, late recurrence was a poor prognostic factor for OS, but the HR for late recurrence was lower than that for early recurrence. When the survival rate after recurrence was analyzed based on the time of recurrence (from the date of recurrence to the date of death or the date of last follow-up) in patients with systemic recurrence, late recurrence was associated with a better prognosis than early recurrence. This suggests that systemic recurrence after 5 years has a more favorable clinical course than early recurrence. Of course, further analysis according to treatment method is needed to confirm these findings.
Patients who underwent neoadjuvant chemotherapy had a significantly lower recurrence rate after 5 years than those who did not undergo chemotherapy or who underwent adjuvant chemotherapy. This may be due to the relatively higher proportion of patients with locally advanced or hormone receptor-negative breast cancer who underwent neoadjuvant chemotherapy. Of the 3 patients with recurrence after 5 years of neoadjuvant chemotherapy, only 1 patient had systemic recurrence. This patient had ypT2N2MO, luminal A disease, had distant metastasis to the lung, pleura, liver, and ovary, and was alive for 30 months after recurrence.
Among the 4 subtypes, luminal B had the highest early and late recurrence rates. The proportion of patients with luminal B disease was especially high in the late recurrence group (63.2%) compared to other subtypes. In addition, unlike other subtypes, late recurrence was a significant prognostic factor for OS only in patients with the luminal B subtype. Late recurrence was very rare in the TNBC and HER2 subtypes, and survival curves of the patients with late recurrence were not significantly different from the survival curves of patients without recurrence. Luminal B breast cancer has different definitions in various studies, but generally it has lower ER expression, higher proliferative gene expression, higher grade and poorer prognosis than luminal A breast cancer [9]. The risk of early recurrence of the luminal B subtype is high [9], and it also has a high risk of recurrence after 5 years. Among ER+ breast cancers, tumors that overexpress HER2 are more likely to fail to endocrine treatment than HER2– tumors [10].
Luminal B is less than 10% responsive to neoadjuvant chemotherapy despite its high proliferation rate [11]. In this study, we defined luminal B as hormone receptor-positive tumors with a high level of Ki-67 or HER2 positivity. Although there was no statistically significant difference in survival curves, HER2+ luminal B seems to have a worse prognosis than luminal B with high Ki-67 level. Patients with late recurrence of HER2+ luminal B breast cancer had a worse prognosis than patients with no recurrence. However, there was no significant difference in OS in luminal B with high Ki-67 level. Previous studies have also shown a poor prognosis for both luminal B with high Ki-67 level and HER2+ luminal B, though HER2+ luminal B seems to have a worse prognosis than luminal B with high Ki-67 level [12].
There might be several reasons for the poor prognosis of patients with the luminal B subtype. There is bidirectional crosstalk between the ER and growth factor receptors (GFR). Various GFR signaling and down-stream pathways including PI3K/AKT/mTOR (phosphoinositide 3-kinase/AKT/mammalian target of rapamycin) and Ras/Raf/MEK/ERK (Ras/Raf/mitogen-activated protein kinase/extracellular signal-regulated kinase), are associated with cancer development, tumor survival, proliferation, migration and metastasis [1314]. One such pathway involves activation of insulin-like growth factor-1R signaling or the fibroblast growth factor signaling pathway. These are related to PR negativity, highly proliferative tumors, and endocrine resistance, and are detected more frequently in the luminal B subtype than in luminal A [1516].
The ER coactivator/coregulator affects endocrine sensitivity/resistance [17]. Osborne et al. [18] showed that high amplified in breast cancer 1 (AIB1) was a good prognostic factor in patients not taking tamoxifen, but it was a poor prognostic factor in patients taking tamoxifen. The AIB1 is a member of the steroid receptor coactivator family, is located on the long arm of chromosome 20 (20q12), and is amplified and overexpressed in breast, ovarian and prostate cancer [19]. AIB1 was found to be overexpressed in about 10% of breast cancers [19] and it plays an essential role in cell growth in vitro and in
vivo [20]. AIB1 also interacts with various signaling molecules mediating cell proliferation, survival, and migration [21]. Many studies suggest the association between AIB1 and HER2 and endocrine resistance in patients with breast cancer treated with tamoxifen, and AIB1 overexpression is associated with HER2 amplification and poor prognosis [182223]. Tumors with relatively high coactivators such as AIB1, particularly HER2 signaling-enhanced tumors capable of activating AIB1, are less responsive to tamoxifen therapy due to the increased estrogen agonist activity of tamoxifen-bound ER [2425]. ER+ HER2+ breast cancer is less likely to respond to tamoxifen than ER+ HER2– breast cancer. The underlying mechanism is not clearly understood, but is thought to be due to ligand-independent activation of the ER by mitogen-activated protein kinase (MAPK) [26]. AIB1 is phosphorylated by MAPK, and high levels of activated AIB1 may reduce the antagonist effect of tamoxifen, particularly in tumors overexpressing HER2 receptors that activate MAPK [27].
We excluded contralateral breast cancer because several factors affect the risk of contralateral breast cancer. Age at diagnosis of primary breast cancer, carriers of susceptibility genes, and antihormonal therapy would be potential competing biases of contralateral breast cancer [28]. Therefore, we could not analyze contralateral breast cancer. This was a potential limitation of this study.
In conclusion, breast cancer has a different recurrence pattern depending on subtype. In the luminal A, TNBC, and HER2 subtypes, early recurrence was associated with poor OS, but late recurrence was not a prognostic factor for OS. However, the luminal B subtype has a high rate of late recurrence and late recurrence was a poor prognostic factor for OS. These findings suggest that further targeted treatments for luminal B breast cancer are needed and patients with this subtype require close long-term surveillance.
Go to : 
Notes
Fund/Grant Support: This research was supported by the National Research Foundation of Korea (NRF2022R1C1C1009101) and Korea University Grant. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Go to : 
References
1. van’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002; 415:530–536. PMID: 11823860.
2. Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011; 5:5–23. PMID: 21147047.
3. Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol. 2013; 31:3083–3090. PMID: 23897954.
4. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010; 28:1684–1691. PMID: 20194857.
5. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007; 13(15 Pt 1):4429–4434. PMID: 17671126.
6. Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009; 27:5700–5706. PMID: 19884543.
7. Montagna E, Bagnardi V, Rotmensz N, Viale G, Renne G, Cancello G, et al. Breast cancer subtypes and outcome after local and regional relapse. Ann Oncol. 2012; 23:324–331. PMID: 21525402.
8. Goldhirsch A, Gelber RD, Price KN, Castiglione M, Coates AS, Rudenstam CM, et al. Effect of systemic adjuvant treatment on first sites of breast cancer relapse. Lancet. 1994; 343:377–381. PMID: 7905550.
9. Tran B, Bedard PL. Luminal-B breast cancer and novel therapeutic targets. Breast Cancer Res. 2011; 13:221. PMID: 22217398.
10. De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005; 11:4741–4748. PMID: 16000569.
11. de Ronde JJ, Hannemann J, Halfwerk H, Mulder L, Straver ME, Vrancken Peeters MJ, et al. Concordance of clinical and molecular breast cancer subtyping in the context of preoperative chemotherapy response. Breast Cancer Res Treat. 2010; 119:119–126. PMID: 19669409.
12. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009; 101:736–750. PMID: 19436038.
13. Fry MJ. Phosphoinositide 3-kinase signalling in breast cancer: how big a role might it play? Breast Cancer Res. 2001; 3:304–312. PMID: 11597319.
14. Hynes NE, Dey JH. Potential for targeting the fibroblast growth factor receptors in breast cancer. Cancer Res. 2010; 70:5199–5202. PMID: 20570901.
15. Creighton CJ, Fu X, Hennessy BT, Casa AJ, Zhang Y, Gonzalez-Angulo AM, et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010; 12:R40. PMID: 20569503.
16. Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010; 70:2085–2094. PMID: 20179196.
17. Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011; 62:233–247. PMID: 20887199.
18. Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003; 95:353–361. PMID: 12618500.
19. Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997; 277:965–968. PMID: 9252329.
20. List HJ, Lauritsen KJ, Reiter R, Powers C, Wellstein A, Riegel AT. Ribozyme targeting demonstrates that the nuclear receptor coactivator AIB1 is a rate-limiting factor for estrogen-dependent growth of human MCF-7 breast cancer cells. J Biol Chem. 2001; 276:23763–23768. PMID: 11328819.
21. Yan J, Tsai SY, Tsai MJ. SRC-3/AIB1: transcriptional coactivator in oncogenesis. Acta Pharmacol Sin. 2006; 27:387–394. PMID: 16539836.
22. Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004; 96:926–935. PMID: 15199112.
23. Alkner S, Jensen MB, Rasmussen BB, Bendahl PO, Fernö M, Rydén L, et al. Prognostic and predictive importance of the estrogen receptor coactivator AIB1 in a randomized trial comparing adjuvant letrozole and tamoxifen therapy in postmenopausal breast cancer: the Danish cohort of BIG 1-98. Breast Cancer Res Treat. 2017; 166:481–490. PMID: 28766132.
24. Smith CL, Nawaz Z, O’Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997; 11:657–666. PMID: 9171229.
25. Takimoto GS, Graham JD, Jackson TA, Tung L, Powell RL, Horwitz LD, et al. Tamoxifen resistant breast cancer: coregulators determine the direction of transcription by antagonist-occupied steroid receptors. J Steroid Biochem Mol Biol. 1999; 69:45–50. PMID: 10418980.
26. Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995; 270:1491–1494. PMID: 7491495.
27. Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000; 20:5041–5047. PMID: 10866661.
28. Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021; 125:601–610. PMID: 34040177.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download