Abstract
Purpose
Postoperative pancreatic fistula (POPF) is the most troublesome complication after pancreaticojejunostomy (PJ). This study aimed to compare the short-term outcomes of 2 different methods of duct-to-mucosa PJ; out-layer continuous suture anastomosis (OCA) and the modified Blumgart method (mBM).
Methods
This retrospective cohort study enrolled patients who underwent curative-intent, open PD between 2015 and 2020. In mBM, 2 transpancreatic U-sutures were performed between the pancreatic margin and jejunum, with reinforced sutures in the central region. Patient demographics, diagnosis, intraoperative factors, postoperative complications, and POPF defined by the International Study Group on Pancreatic Fistula were investigated. Clinically relevant POPF (CR-POPF) included grades B and C POPF.
Results
A total of 184 patients underwent OCA, and 96 patients underwent mBM. The mBM group had more patients who underwent neoadjuvant therapy. The fistula risk scores were comparable between the 2 groups. Both groups showed no significant differences in CR-POPF and overall surgical complication rates. The total operation time was comparable, although the operation time for PJ was shorter in mBM.
Pancreaticoduodenectomy (PD) is a technically demanding procedure. PD is associated with various complications, such as postoperative pancreatic fistula (POPF), delayed gastric emptying (DGE), postpancreatectomy hemorrhage, bile leakage, chyle leakage, intraabdominal abscess, and wound infection or dehiscence [12]. Among these, POPF is a major threat for patients and can result in intraabdominal abscesses, leading to sepsis or pseudoaneurysm, ultimately causing severe hemorrhage [13]. POPF is an abnormal communication between the pancreatic ductal epithelium and an epithelial surface containing a pancreas-derived, enzyme-rich fluid. Consequently, it can increase medical costs due to longer hospital stays, readmissions, and mortality [3]. POPF is thought to occur in 3%–45% of pancreatic operations in high-volume centers [1].
There have been attempts to reduce the occurrence of POPF by applying various techniques, but the results are still controversial [2456789101112131415161718192021222324]. Pancreaticojejunostomy (PJ), which anastomoses the pancreatic stump to the jejunal loop, was popularized by Whipple in the 1940s [25]. Currently, the duct-to-mucosa technique is widely used in high-volume centers [17]. In this technique, the pancreatic stump is anastomosed to the jejunal loop, but the main pancreatic duct (MPD) is sutured directly to the opening created on the side of the jejunal loop. It can be subcategorized into 2 groups, described by an out-layer suture of the pancreatic capsule (either interrupted or continuous suture) or a transpancreatic U-shaped suture. In the transpancreatic methods, such as the Blumgart method or the Kakita method, the pancreatic parenchyma is penetrated in full-thickness and sutured to the seromuscular layer of the jejunal loop, both dorsally and ventrally [710]. In some studies, better results have been seen in the modified Blumgart method (mBM) compared to other duct-to-mucosa anastomoses [59101114151620]. However, results have been inconsistent [91014]. Therefore, in this study, we aimed to compare the perioperative outcomes between out-layer continuous anastomosis (OCA) and mBM.
This study was approved by the Institutional Review Board in Seoul National University Hospital (No. 2105-214-122). It was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to the retrospective nature.
This was a retrospective cohort study with prospectively collected medical data, including complications and grades. Patients undergoing curative-intent, open PD with periampullary disease between February 2015 and November 2020 in our hospital were included in this study, and all patients were aged 18 years and above. All operations were performed in elective surgery by 1 senior hepato-pancreato-biliary surgeon who had already performed over 1,000 pancreatectomies. He had performed the OCA between 2015 to December 2018, and he has performed the mBM since January 2019. Patients who underwent a combined resection of other organs or parts of the pancreas other than the pancreatic head, without internal drainage to the MPD, and with a previous history of pancreatic resection and pancreaticogastrostomy (PG) were excluded.
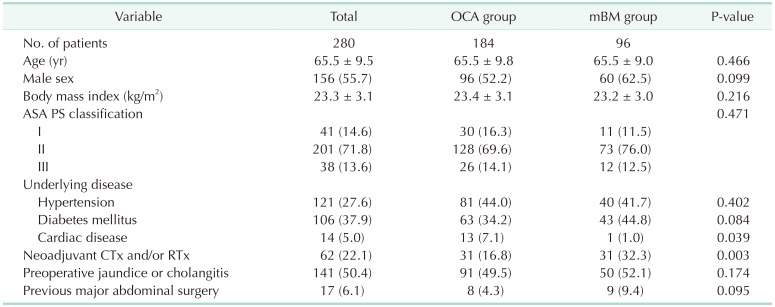
Clinical variables included patient demographics (age, sex, body mass index [BMI], American Society of Anesthesiologists physical status classification, underlying diseases including hypertension, diabetes mellitus, cardiac disease, respiratory disease, neoadjuvant chemotherapy and/or radiation therapy, previous major abdominal surgery), jaundice or cholangitis, diagnosis related to pancreatic disease or malignancy, intraoperative factors (operative procedure, superior mesenteric vein and/or portal vein resection, texture of remnant pancreas, MPD size, duration of operation, operation time for PJ, estimated blood loss [EBL], type of PJ [OCA or mBM]), and postoperative complications, including POPF [2627]. If the jaundice or cholangitis existed preoperatively, endoscopic retrograde bililary drainage or percutaneous transhepatic biliary drainage were performed as surgeon’s preference. The pancreatic texture was determined by the surgeon during the operation. The MPD diameter was measured on the cross-sectional image of preoperative CT image. The risk of fistula was measured and categorized using the alternative fistula risk score (aFRS) developed by Mungroop et al. [28].
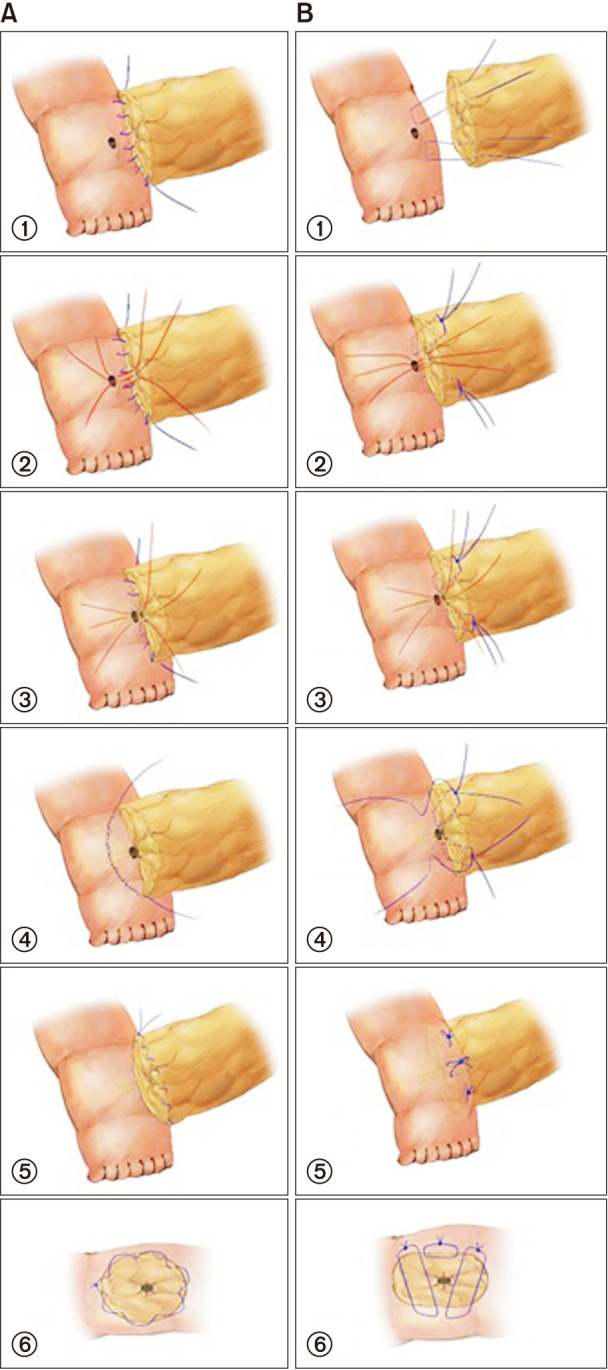
After placing the pancreatic stump and jejunal loop, the MPD exposed on the resection surface was sutured with 5-0 polydioxanone (PDS II, Ethicon, Somerville, NJ, USA) in 6 different directions from the duct to the resection surface for anastomosis preparation. The outer layer of the PJ on the dorsal side was performed by suturing the pancreatic capsule at the edge of the resection surface to the seromuscular layer of the jejunal loop with 4-0 polypropylene (Surgipro, Covidien, Mansfield, MA, USA). Among the previously prepared sutures, 3 dorsal-side sutures were anastomosed to the jejunal opening in full-thickness, which was opened at the size of the MPD by electrocautery. Internal drainage was placed in the duct-to-mucosa anastomosis site with a 1–2 mm diameter silastic polyethylene tube. The ventral side of the inner layer was sutured with the 3 remaining sutures; the external layer was sutured using the continuous method. A Jackson-Pratt (JP) drain was located near both the superior and inferior sides of the PJ site (Fig. 1A).
Instead of suturing the outer layer, a transpancreatic U-suture was performed in the mBM. The suture was inserted 5–8 mm distally from the cut surface of the pancreatic stump, penetrating from the ventral to the dorsal side, in full thickness of the pancreas using 4-0 polypropylene (Surgipro). Next, the seromuscular layer of the jejunal loop was sutured with the same stitch; the stitch penetrated the pancreatic stump back from the dorsal to the ventral side. Avoiding injury of the MPD, each of the 2 transpancreatic U-sutures was performed on the superior and inferior borders of the PJ, tied but left uncut. Duct-to-mucosa anastomosis was performed in the same manner as OCA using interrupted suture with 5-0 polydioxanone (PDS II) in 6 different directions, with the insertion of an internal stent. With the uncut transpancreatic U-suture, the ventral side of the jejunal loop was sutured in the seromuscular layer, concealing the resection surface of the pancreatic stump. Finally, an interrupted, suture-reinforcing PJ was performed between 2 transpancreatic U-sutures in the central and ventral parts. The JP drain was located near the superior and inferior sides of the PJ site (Fig. 1B).
The amylase concentration of the drainage fluid was measured on postoperative days (PODs) 1, 3, and 5. Postoperative CT was performed on POD 4. If the amylase level was less than 3 times the upper normal serum value and no definite fluid collection was found on CT scan, the JP drain was removed. If fluid collection was seen on CT scan, the JP drain was repositioned for proper drainage. Somatostatin analogs were not routinely used.
POPF was defined by the International Study Group of Pancreatic Fistula [1]. Clinically relevant POPF (CR-POPF) was defined as either grade B or C. Other surgical complications included DGE, bleeding, intraabdominal fluid collection or abscess, wound infection, anastomotic stenosis, bile leakage, chyle leakage, and ileus [12]. Cardiac and/or respiratory events were evaluated as nonsurgical complications. Severe complications were defined as a Clavien-Dindo classification of ≥III. Reoperation and mortality were counted in cases that occurred within 90 days after surgery. Major abdominal surgery was defined as operations that included at least 1 gastrointestinal anastomosis or involving parenchymal resection of the pancreas or liver [29].
All statistical analyses were performed using IBM SPSS Statistics ver. 22.0 (IBM Corp., Armonk, NY, USA). Nominal data were compared using the chi-square test or the Fisher exact test; continuous data were examined using the Student t-test. Continuous variables are expressed as mean ± standard deviation. Statistical significance was set at P < 0.05. Risk factors for CR-POPF, as well as surgical and severe complications, were identified using univariate and multivariate analyses. Variables found to be associated with complications in the univariate analysis (P < 0.05) were entered into a stepwise logistic regression model for multivariate analysis of risk factors.
A total of 280 patients with periampullary disease underwent PD. OCA was performed in 184 patients (65.7%), and mBM was performed in 96 patients (34.3%). The mean age was 65.5 years, and the mean BMI was 23.3 kg/m2. Of the patients, 55.7% were men. Furthermore, 22.1% of the patients underwent neoadjuvant chemotherapy and/or radiation therapy, wherein more patients in the mBM group underwent neoadjuvant therapy than did those in the OCA group (16.8% vs. 32.3%, P = 0.003). The incidence of jaundice or cholangitis were comparable between the OCA and mBM group (49.5% vs. 52.1%, P = 0.174), and there was no significant difference of method for solving them between 2 groups. Detailed information on patient demographics is provided in Table 1.
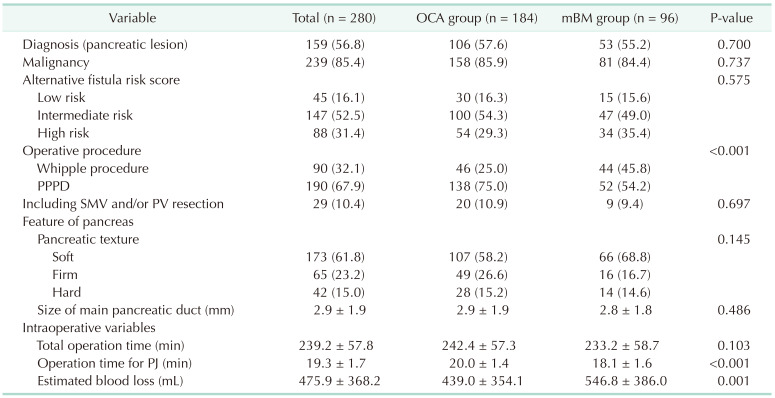
The diagnosis was similar in the OCA and mBM groups (57.6% vs. 55.2%, P = 0.700). In terms of pancreatic features, no significant difference was found in pancreatic texture (soft, 58.2% vs. 68.8%, P = 0.145) and MPD size (2.9 mm vs. 2.8 mm, P = 0.486). When the risk of POPF was evaluated using aFRS, no difference in fistula risk was observed (Table 2).
For intraoperative variables, operative time for PJ was shorter in the mBM group (20.0 minutes vs. 18.1 minutes, P < 0.001), but the total operative time was comparable (242.4 minutes vs. 233.2 minutes, P = 0.103). This was thought to be because there were more patients who underwent neoadjuvant therapy due to advanced pancreatic cancer in the mBM group. There was more EBL in the mBM group (439.0 mL vs. 546.8 mL, P = 0.001) (Table 2).
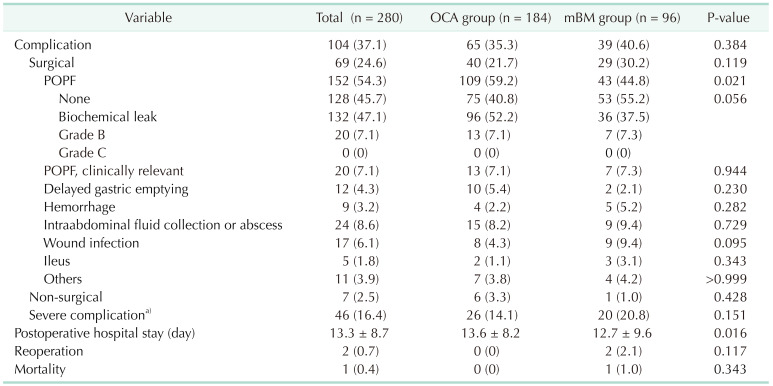
The overall postoperative complication rate was 37.1%, including both surgical and nonsurgical complications (Table 3). The overall CR-POPF rate was 7.1%; there was no grade C in this study population. POPF was significantly lower in the mBM group (59.2% vs. 44.8%, P = 0.021). Although CR-POPF was not significantly different between the OCA and mBM groups (7.1% vs. 7.3%, P = 0.944), there were significantly fewer patients with biochemical leak in the mBM group (52.2% vs. 37.5%, P = 0.016). Patients with surgical complications (21.7% vs. 30.2%, P = 0.119) or severe complications with Clavien-Dindo grade III or more (14.1% vs. 20.8%, P = 0.151) were comparable. Postoperative hospital stay was shorter in the mBM group (13.6 days vs. 12.7 days, P = 0.016). There were 2 cases of reoperation in the mBM group due to bleeding (stump of the right gastroepiploic artery and small artery of the transverse colon mesentery). In the mBM group, there was 1 case of mortality due to liver failure secondary to parenchymal necrosis by infarction, leading to multiorgan failure.
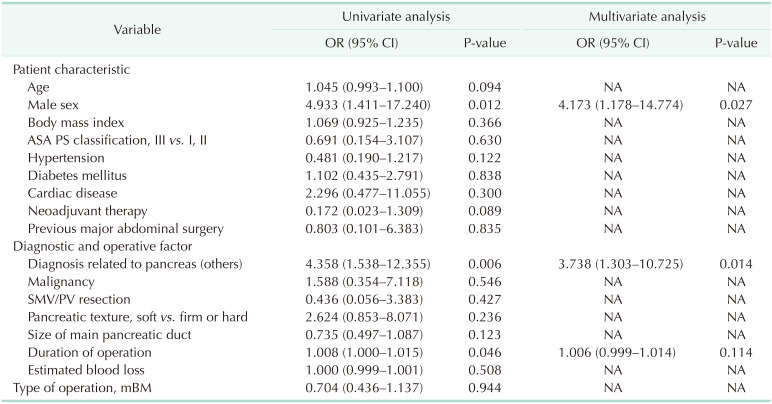
Logistic regression analysis was performed to investigate the factors associated with postoperative outcomes (Table 4). In the univariate analysis, male sex (P = 0.012), diagnosis not related to pancreas (P = 0.006), and duration of operation (P = 0.046) were significant factors for CR-POPF. In the multivariate analysis, male sex (odds ratio [OR], 4.173; 95% confidence interval [CI], 1.178–14.774; P = 0.027) and diagnosis not related to pancreas (OR, 0.374; 95% CI, 1.303–10.725; P = 0.014) were independent risk factors for CR-POPF. In terms of severe complications after PD, both diagnoses not related to the pancreas (OR, 3.311; 95% CI, 1.834–5.977; P < 0.001) and duration of surgery (OR, 1.008; 95% CI, 1.003–1.013; P = 0.003) were significant factors related to severe complications (Supplementary Table 1). None of these postoperative outcomes was related to the PJ technique.
As POPF is the most threatening post-PD complication, many attempts have been made to reduce its occurrence [2317]. Previous studies have investigated the rate of POPF when placing an internal or external stent; comparable results were shown in a recent randomized study [1923]. In other studies, the location of the anastomosis of the pancreatic stump in the jejunum (PJ) or stomach (PG) was compared, and no difference was found [46]. When performing PJ, either the invagination technique of the pancreatic stump or the duct-to-mucosal anastomosis technique can be used; however, no technique has revealed itself to be superior [2124]. Various modifications of surgical techniques have been applied according to surgeons’ preferences, including the Blumgart method [578910111314161820]. Although none of them have been found to be superior to another, new attempts to reduce POPF are still ongoing, such as applying polyglycolic acid or fibrinogen/thrombin-coated collagen patch [1231222].
In a recent systematic review, Li et al. [15] analyzed 11 studies comparing the Blumgart method with other PJs. The Blumgart method showed a significantly lower rate of postoperative complications, including POPF. However, the details of the Blumgart method differed from each other in the included studies. Moreover, PJ techniques, compared to the Blumgart method, the use of stents, and abdominal lavage were also applied differently in each study. Most importantly, these studies were mostly retrospective (10 of 11). Thus, further well-designed studies are required to minimize the confounding factors.
Compared to the original Blumgart method, our modification has its main feature in reducing the number of transpancreatic U-sutures, from at least 4 to just 2. As a result, it was possible to significantly reduce the operation time for PJ, compared to OCA (20.0 minutes vs. 18.1 minutes, P < 0.001) with comparable postoperative outcomes. This might contribute to the short learning curve of this technique, as it can be easily taught and applied, which can be a major advantage in performing PJ [711]. As mBM is less time-consuming and simpler, it is applied when robotic PD is performed in our center [30]. Additionally, the theoretical principle of reducing POPF using the Blumgart method is that it not only covers the cut surface of the pancreatic stump but also provides a compression effect to the pancreatic parenchyma, preventing oozing of pancreatic juice that occurs from the minor duct on the cut surface. The prevalence of biochemical leak was significantly lower in the mBM group (59.2% vs. 44.8%, P = 0.021). However, there was no significant difference in the rate of CR-POPF between the OCA and mBM groups (7.1% vs. 7.3%, P = 0.944). In addition, the PJ type was not shown to be a significant factor for postoperative outcomes in the multivariate analysis, as POPF, mBM, and OCA were comparable (Table 4). In the same manner, PJ type was not related to severe complications that were classified as Clavien-Dindo grade III or higher.
Even though there were no statistically significant differences in severe complications, the percentage was higher in the mBM group. As previously mentioned, neoadjuvant therapy was administered to more patients in the mBM group. Some studies have reported that neoadjuvant therapy is associated with a lower rate of POPF. However, complications other than POPF could have been induced by tissue injury caused by more dissection of adhesions and neoadjuvant therapy in the advanced stage. Thus, it might have resulted in a slightly higher occurrence of complications, such as intraabdominal abscess (5.4% vs. 8.3%, P = 0.348) or fluid collection and wound complications (2.7% vs. 6.3%, P = 0.195).
Our study has some limitations. First, this was a retrospective study with possible confounding factors. However, all PJ procedures were performed by 1 hepato-pancreato-biliary surgeon in a consistent surgical technique with equal proficiency using the same suture material, minimizing discrepancies that can occur during surgery. Moreover, since there was no disparity between the OCA and mBM groups with no significant difference in fistula risk scores (Fig. 1), it is likely that selection bias was minimized. Second, the cases were not contemporaneous as this was a before-and-after study. Although a learning curve was not thought to be required, it could have an impact on the results. However, a skilled surgeon with ample experience performed the surgery; thus, surgical results are expected to be consistent. Third, all PDs in this study were performed by a single surgeon in a single institution. Thus, there are limitations in the generalizability of the results.
In conclusion, no significant differences were observed in postoperative outcomes between the OCA and mBM groups, but the operation time for PJ in mBM was shorter. Since mBM is a safe, relatively simple, and time-saving technique, it can be a good choice when duct-to-mucosa PJ is performed.
References
1. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017; 161:584–591. PMID: 28040257.
2. Shrikhande SV, Sivasanker M, Vollmer CM, Friess H, Besselink MG, Fingerhut A, et al. Pancreatic anastomosis after pancreatoduodenectomy: a position statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2017; 161:1221–1234. PMID: 28027816.
3. Pedrazzoli S. Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): a systematic review and analysis of the POPF-related mortality rate in 60,739 patients retrieved from the English literature published between 1990 and 2015. Medicine (Baltimore). 2017; 96:e6858. PMID: 28489778.
4. Andrianello S, Marchegiani G, Malleo G, Masini G, Balduzzi A, Paiella S, et al. Pancreaticojejunostomy with externalized stent vs pancreaticogastrostomy with externalized stent for patients with high-risk pancreatic anastomosis: a single-center, phase 3, randomized clinical trial. JAMA Surg. 2020; 155:313–321. PMID: 32101272.
5. Casadei R, Ricci C, Ingaldi C, Alberici L, De Raffele E, Minni F. Comparison of Blumgart anastomosis with duct-to-mucosa anastomosis and invagination pancreaticojejunostomy after pancreaticoduodenectomy: a single-center propensity score matching analysis. J Gastrointest Surg. 2021; 25:411–420. PMID: 31997074.
6. Grendar J, Ouellet JF, Sutherland FR, Bathe OF, Ball CG, Dixon E. In search of the best reconstructive technique after pancreaticoduodenectomy: pancreaticojejunostomy versus pancreaticogastrostomy. Can J Surg. 2015; 58:154–159. PMID: 25799130.
7. Grobmyer SR, Kooby D, Blumgart LH, Hochwald SN. Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg. 2010; 210:54–59. PMID: 20123332.
8. Hall RI, Rhodes M, Isabel-Martinez L, Kelleher J, Venables CW. Pancreatic exocrine function after a sutureless pancreatico-jejunostomy following pancreaticoduodenectomy. Br J Surg. 1990; 77:83–85. PMID: 2302521.
9. Hirono S, Kawai M, Okada KI, Miyazawa M, Kitahata Y, Hayami S, et al. Modified Blumgart mattress suture versus conventional interrupted suture in pancreaticojejunostomy during pancreaticoduodenectomy: randomized controlled trial. Ann Surg. 2019; 269:243–251. PMID: 29697455.
10. Kawakatsu S, Inoue Y, Mise Y, Ishizawa T, Ito H, Takahashi Y, et al. Comparison of pancreatojejunostomy techniques in patients with a soft pancreas: Kakita anastomosis and Blumgart anastomosis. BMC Surg. 2018; 18:88. PMID: 30355352.
11. Kojima T, Niguma T, Watanabe N, Sakata T, Mimura T. Modified Blumgart anastomosis with the “complete packing method” reduces the incidence of pancreatic fistula and complications after resection of the head of the pancreas. Am J Surg. 2018; 216:941–948. PMID: 29606278.
12. Kwon J, Shin SH, Lee S, Park G, Park Y, Lee SJ, et al. The effect of fibrinogen/thrombin-coated collagen patch (TachoSil®) application in pancreaticojejunostomy for prevention of pancreatic fistula after pancreaticoduodenectomy: a randomized clinical trial. World J Surg. 2019; 43:3128–3137. PMID: 31502003.
13. Lee SE, Yang SH, Jang JY, Kim SW. Pancreatic fistula after pancreaticoduodenectomy: a comparison between the two pancreaticojejunostomy methods for approximating the pancreatic parenchyma to the jejunal seromuscular layer: interrupted vs continuous stitches. World J Gastroenterol. 2007; 13:5351–5356. PMID: 17879405.
14. Lee YN, Kim WY. Comparison of Blumgart versus conventional duct-to-mucosa anastomosis for pancreaticojejunostomy after pancreaticoduodenectomy. Ann Hepatobiliary Pancreat Surg. 2018; 22:253–260. PMID: 30215047.
15. Li Z, Wei A, Xia N, Zheng L, Yang D, Ye J, et al. Blumgart anastomosis reduces the incidence of pancreatic fistula after pancreaticoduodenectomy: a systematic review and meta-analysis. Sci Rep. 2020; 10:17896. PMID: 33087777.
16. Menonna F, Napoli N, Kauffmann EF, Iacopi S, Gianfaldoni C, Martinelli C, et al. Additional modifications to the Blumgart pancreaticojejunostomy: results of a propensity score-matched analysis versus Cattel-Warren pancreaticojejunostomy. Surgery. 2021; 169:954–962. PMID: 32958267.
17. Olakowski M, Grudzińska E, Mrowiec S. Pancreaticojejunostomy: a review of modern techniques. Langenbecks Arch Surg. 2020; 405:13–22. PMID: 31975148.
18. Peng SY, Wang JW, Li JT, Mou YP, Liu YB, Cai XJ. Binding pancreaticojejunostomy: a safe and reliable anastomosis procedure. HPB (Oxford). 2004; 6:154–160. PMID: 18333069.
19. Qureshi S, Ghazanfar S, Quraishy MS, Rana R. Stented pancreatico-duodenectomy: does it lead to decreased pancreatic fistula rates?: a prospective randomized study. J Pak Med Assoc. 2018; 68:348–352. PMID: 29540866.
20. Satoi S, Yamamoto T, Yanagimoto H, Yamaki S, Kosaka H, Hirooka S, et al. Does modified Blumgart anastomosis without intra-pancreatic ductal stenting reduce post-operative pancreatic fistula after pancreaticojejunostomy? Asian J Surg. 2019; 42:343–349. PMID: 30087009.
21. Senda Y, Shimizu Y, Natsume S, Ito S, Komori K, Abe T, et al. Randomized clinical trial of duct-to-mucosa versus invagination pancreaticojejunostomy after pancreatoduodenectomy. Br J Surg. 2018; 105:48–57. PMID: 29265404.
22. Shibuya K, Jang JY, Satoi S, Sho M, Yamada S, Kawai M, et al. The efficacy of polyglycolic acid felt reinforcement in preventing postoperative pancreatic fistula after pancreaticojejunostomy in patients with main pancreatic duct less than 3 mm in diameter and soft pancreas undergoing pancreatoduodenectomy (PLANET-PJ trial): study protocol for a multicentre randomized phase III trial in Japan and Korea. Trials. 2019; 20:490. PMID: 31399139.
23. Shin YC, Jang JY, Chang YR, Jung W, Kwon W, Kim H, et al. Comparison of long-term clinical outcomes of external and internal pancreatic stents in pancreaticoduodenectomy: randomized controlled study. HPB (Oxford). 2019; 21:51–59. PMID: 30093143.
24. Singh AN, Pal S, Mangla V, Kilambi R, George J, Dash NR, et al. Pancreaticojejunostomy: does the technique matter?: a randomized trial. J Surg Oncol. 2018; 117:389–396. PMID: 29044532.
25. Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg. 1935; 102:763–779. PMID: 17856666.
26. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213. PMID: 15273542.
27. Knuf KM, Maani CV, Cummings AK. Clinical agreement in the American Society of Anesthesiologists physical status classification. Perioper Med (Lond). 2018; 7:14. PMID: 29946447.
28. Mungroop TH, van Rijssen LB, van Klaveren D, Smits FJ, van Woerden V, Linnemann RJ, et al. Alternative fistula risk score for pancreatoduodenectomy (a-FRS): design and international external validation. Ann Surg. 2019; 269:937–943. PMID: 29240007.
29. Heger P, Probst P, Wiskemann J, Steindorf K, Diener MK, Mihaljevic AL. A systematic review and meta-analysis of physical exercise prehabilitation in major abdominal surgery (PROSPERO 2017 CRD42017080366). J Gastrointest Surg. 2020; 24:1375–1385. PMID: 31228083.
30. Kim HS, Han Y, Kang JS, Kim H, Kim JR, Kwon W, et al. Comparison of surgical outcomes between open and robot-assisted minimally invasive pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2018; 25:142–149. PMID: 29117639.
SUPPLEMENTARY MATERIALS
Supplementary Table 1 can be found via https://doi.org/10.4174/astr.2022.103.6.331.
Supplementary Table 1
Univariate and multivariate analyses for severe complications (Clavien-Dindo grade ≥ III)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download