Abstract
Purpose
This study was performed to evaluate the utility of the fistula risk score (FRS) and its components in predicting the occurrence of postoperative pancreatic fistula and other significant postoperative complications after resections of pancreatic neuroendocrine tumors.
Methods
Retrospective analysis of 131 patients operated on for pancreatic neuroendocrine tumors between 2015 and 2021 was performed. The correlation of the FRS scale with the occurrence of postoperative pancreatic fistulas and postoperative complications according to the Clavien-Dindo classification was analyzed; only in 109 cases of distal resections and pancreatoduodenectomies (PD).
Results
Soft pancreatic texture and intraoperative blood loss of >700 mL are risk factors for clinically significant pancreatic fistula (P = 0.001 and P = 0.001, respectively) and significant postoperative complications (P = 0.016 and P = 0.001, respectively). Wirsung duct diameter (WDD) was associated only with the occurrence of postoperative pancreatic fistula (P = 0.013). FRS scale is associated with the occurrence of pancreatic fistulas and clinically significant postoperative complications in cases of distal resections and PDs (P < 0.001 and P = 0.005, respectively). Postoperative complications are correlated with the occurrence of fistula type B or C (P < 0.001).
Conclusion
Soft pancreatic texture, intraoperative blood loss of >700 mL, and a WDD of ≤3 mm are risk factors for clinically significant postoperative pancreatic fistula. FRS may be applied not only in PDs but also in distal pancreatectomies. Unfortunately, it is not used in total pancreatic resections and enucleations since FRS takes into account the WDD.
Pancreatic resections are associated with a high risk of postoperative surgical complications [1]. According to the reviewed literature, they may affect up to 50% of patients [1]. Resection of pancreatic neuroendocrine tumors (NETs) carries a higher risk of postoperative complications compared to resections of adenocarcinomas and inflammatory tumors [2]. One of the most common complications after pancreatic resections for NETs is pancreatic fistula occurring in 5%–30% of cases [34]. The International Study Group of Pancreatic Fistula (ISGPF) has systematized the division and definition of pancreatic fistulas [5]. The clinically relevant fistulas are of types B and C, as they require intervention and treatment [5]. Furthermore, type C pancreatic fistulas are associated with a high risk of patient death [5].
This is particularly important in NETs, as some of these tumors can be subjected to observation and do not require immediate resection (tumors that meet the criteria defined by the European Neuroendocrine Tumor Society) [6]. This applies to asymptomatic NETs of 1–2 cm, in which decisions on whether to perform resection or not are made for each individual patient [6]. The use of the fistula risk score (FRS) scale and the determination of its association with postoperative pancreatic fistula and severe complications on the Clavien-Dindo grade (≥III) can also facilitate decisions regarding the use of early prophylaxis of complications in the postoperative period in resected patients.
Knowledge of risk factors of postoperative complications allows for better planning of the surgery and reduces their incidence of complications. This applies to pancreatoduodenectomy, distal pancreatectomy, and enucleation as well as total pancreatectomy [78]. According to some authors, risk factors for postoperative complications include age, chronic diseases, and obesity [89]. Risk factors for postoperative pancreatic fistula include soft pancreatic texture, intraoperative blood loss, and Wirsung duct diameter of <3 mm, as well as the histopathologic type of resected tumor [910], which are all included in the FRS used to predict the occurrence of postoperative pancreatic fistula [10]. It is a scale validated mainly in pancreatoduodenectomies performed for pancreatic adenocarcinoma [11]. The rarity of pancreatic NETs (1–2/100,000 people) [12], as well as a relatively small number of publications regarding the use of the FRS scale in NET resections, prompted us to evaluate the relationship between this scale and the occurrence of postoperative complications in pancreatic NETs.
The aim of this study was to evaluate the utility of the FRS scale and the significance of individual risk factors included in it in predicting the occurrence of postoperative pancreatic fistulas which were assessed according to the ISGPF classification, as well as other major postoperative complications according to the Clavien-Dindo classification after resections of pancreatic NETs.
This was a retrospective study of medical records and all data had been fully anonymized before we accessed them. Written informed consent was obtained from all participants. All procedures were in accordance with the 1964 Helsinki declaration regarding medical research involving human subjects and its later amendments or comparable ethical standards. Our retrospective analysis of patients’ medical records fell into a category exempt from Institutional Review Board approval according to local laws.
A retrospective analysis of 131 patients operated on for pancreatic NETs between 2015 and 2021 at the Department of Gastrointestinal Surgery of University Clinical Center in Katowice was performed. The analysis included pancreatoduodenectomies, distal pancreatectomies, total pancreatectomies, and enucleations. In order to standardize the study group, all analyzed procedures were performed by laparotomy. Clinical data (age, sex) of patients, data from operation protocols (type of surgery performed, intraoperative blood loss, pancreatic texture, diameter of Wirsung duct, and location of tumor within the pancreas), and the correlation of FRS scale with the occurrence of postoperative pancreatic fistulas assessed according to ISGPF classification [5], as well as postoperative complications assessed according to the Clavien-Dindo classification [13], were analyzed.
Type B and C pancreatic fistulas were considered clinically significant, as they require treatment [5]. Complications of grade III and above on the Clavien-Dindo classification were considered significant. The pancreatic texture was assessed during intraoperative examination by the operating surgeon. Assessment of pancreatic texture is subjective. However, when performed by a surgeon with extensive experience in pancreatic surgery, it is considered a reliable and accepted method according to studies [14]. A small Wirsung duct was defined based on its diameter (≤3 mm). Conversely, if the pancreatic duct diameter was >3 mm, it was considered dilated. Intraoperative blood loss was divided into 3 groups (≤400 mL, 401–700 mL, and 701–1,000 mL) [10].
The FRS scale is used to assess the risk of pancreatic fistula. The number of points obtained on the FRS scale allows the patient to be classified in the appropriate pancreatic fistula risk group [10]: 0 point, insignificant; 1–2 points, low-risk group; 3–6 points, medium risk group; and 7–10 points, high-risk group [10].
From the analyses regarding the association of the risk of pancreatic fistula according to the FRS scale with the actual occurrence of pancreatic fistula, enucleation procedures (lack of possibility of intraoperative assessment of the diameter of the pancreatic duct), as well as total pancreatectomies (the entire organ is removed and therefore there are no pancreatic fistulas postoperatively) were excluded.
A descriptive analysis was performed. A 95% confidence interval (CI) was used and the distribution of quantitative variables was analyzed. In case of variables with distribution close to normal, their mean with standard deviation was given; in cases of distribution different from normal, their median with interquartile range was given. Correlation analysis was performed between clinical parameters and postoperative complications, where P < 0.05 was considered statistically significant. The chi-square test or Fischer exact test when needed was used during the analysis of nominal variables. The strength of correlation was calculated using the Phi coefficient for 2 × 2 tables or Cramer’s V for larger ones. Correlations between quantitative and qualitative variables were calculated using the Eta test (for calculating correlation coefficient) and point biserial correlation (for calculating P-value). Multivariate, binary regression analysis was performed. All calculations, as well as statistical analysis, were performed in IBM SPSS Statistics ver. 26 (IBM Corp., Armonk, NY, USA).
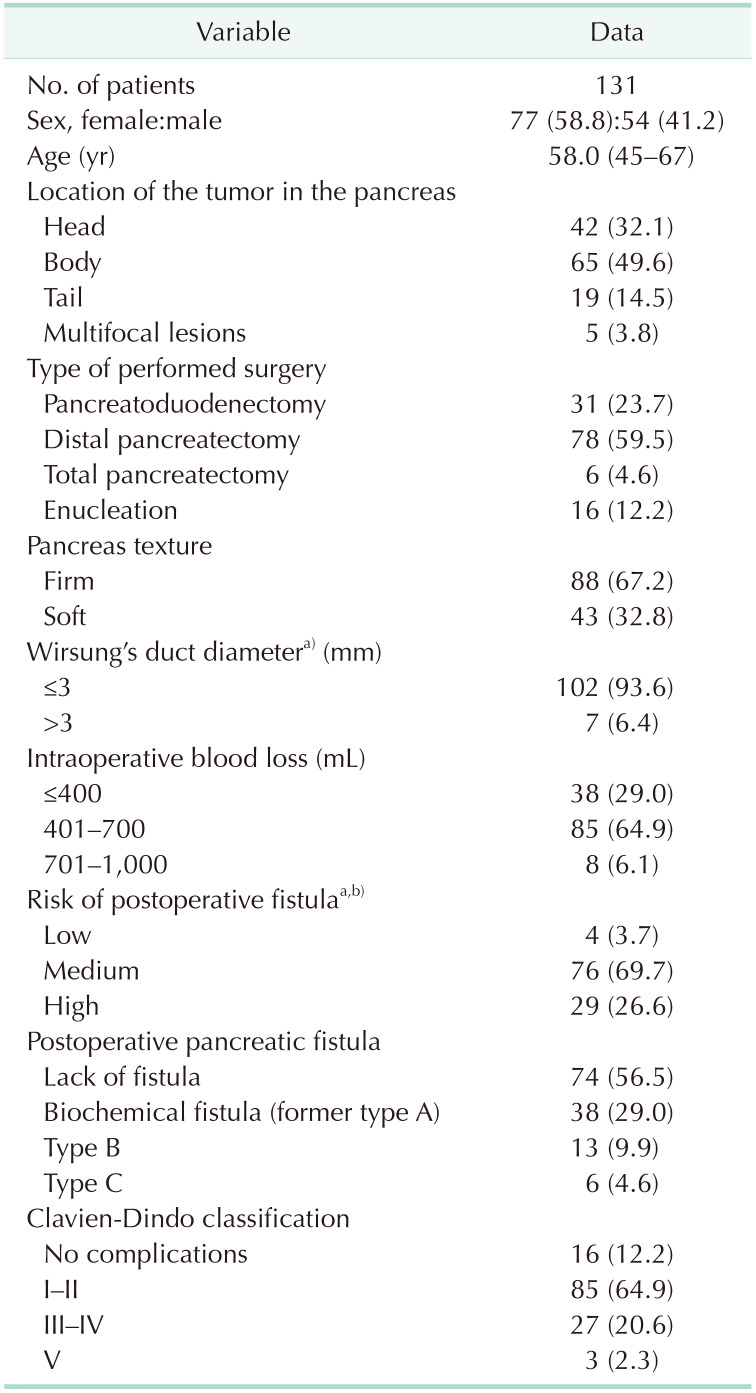
The analysis included 77 female (58.8%) and 54 male patients (41.2%). The median age of the patients was 58 years. Among the operated patients, 65 (49.6%) had tumors located in the body of the pancreas, 42 (32.1%) in the head, 19 (14.5%) in the tail, and in 5 cases (3.8%) the lesions were multifocal and located throughout the organ. In the case of multifocal lesions, all patients had a diagnosis of multiple endocrine neoplasia type 1 syndrome.
Of the total procedures performed, there were 78 distal pancreatectomies (59.5%), 31 pancreatoduodenectomies (23.7%), 16 enucleations (12.2%), and 6 total pancreatectomies (4.6%). All procedures (100%) were performed by laparotomy. Among the patients included in the analysis (total pancreatectomies were excluded), pancreatic fistulas occurred in 57 cases (43.5%). Thirty-eight patients (29.0%) had type A fistulas (biochemical); 13 (9.9%) had type B fistulas; and 6 (4.6%) had type C fistulas. Clinically significant pancreatic fistula occurred in 3 cases of pancreatoduodenectomy (9.7%), 15 cases of distal resection (19.2%), and 1 enucleation (6.3%). In cases of other surgical complications that were clinically significant according to the Clavien-Dindo classification, 3 pancreatoduodenectomy pancreatic anastomosis failures (9.7%), 2 biliary anastomosis failures (6.5%), and 1 gastrointestinal anastomosis (3.2%) occurred. Biliary anastomosis failure also occurred in 2 patients (33.3%) undergoing total pancreatectomy. An intraabdominal abscess was found in 2 patients (6.5%) after pancreatoduodenectomy and 3 (3.8%) after distal resection. Postoperative acute pancreatitis occurred in 1 case of pancreatoduodenectomy (3.2%), and 2 (2.6%) after peripheral pancreatic resection. Postoperative hemorrhage occurred in 1 patient (1.3%) after distal, and in 2 patients (33.3%) after total pancreatectomy. In 3 cases (2.3%), patient death occurred—2 times after pancreatoduodenectomy (one for surgical reasons [pancreatic anastomosis failure complicated by peritonitis] and another because of respiratory failure during multiorgan failure), and 1 after total pancreatectomy (due to multiorgan failure). The characteristics of patient intraoperative as well as postoperative data of the analyzed group are presented in Table 1.
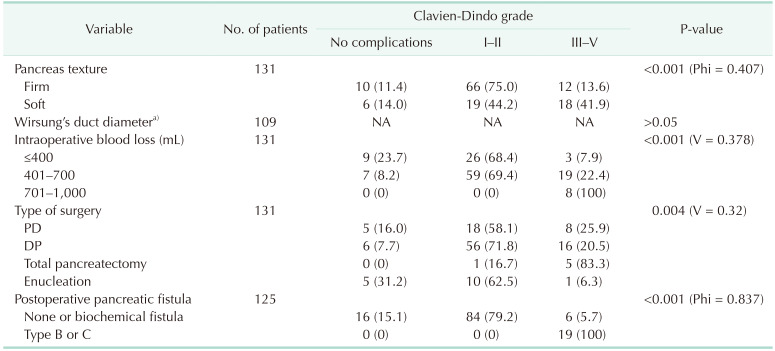
The relationship of pancreatic fistula occurrence with pancreatic texture was established. Type B or C fistula occurred in 4 cases (4.7%) of firm pancreatic texture and in 15 cases (37.5%) in which the pancreas was described as soft (P < 0.001, Phi = 0.447, moderate correlation strength). A correlation between clinically relevant complications and pancreatic texture was found. The soft pancreatic texture was associated with a higher incidence of clinically significant complications according to the Clavien-Dindo classification (P < 0.001, Phi = 0.407, moderate correlation strength). Of the 43 cases in which the pancreas was defined as soft, 18 (41.9%) were associated with clinically significant complications. The above-mentioned correlations are presented in Table 2.
The association of Wirsung duct diameter with the occurrence of clinically significant pancreatic fistulas was also evaluated. The pancreatic duct was associated with type B and C fistulas (P = 0.013, Eta = 0.216, weak correlation). There was no association between Wirsung duct diameter and the Clavien-Dindo grade of postoperative complications (P > 0.05) (Table 3).
As the amount of intraoperative blood loss increases, the incidence of clinically relevant pancreatic fistulas increases (P = 0.019, Phi = 0.316, moderate strength of correlation). Higher intraoperative blood loss is associated with higher severity of postoperative complications on the Clavien-Dindo classification (P < 0.001, Cramer’s V = 0.378, moderate correlation strength) (Tables 2, 3).
In addition, the occurrence of the pancreatic fistula is significantly affected by the type of surgical procedure. Clinically relevant pancreatic fistulas are almost 2 times more frequent in distal pancreatectomies compared to pancreatoduodenectomies. There was no statistically significant correlation between the type of surgery and the type of pancreatic fistula (P > 0.05). In 1 case (6.3%), a clinically significant pancreatic fistula occurred after enucleation.
The type of performed surgery also influences the rate of surgical complications according to the Clavien-Dindo classification. Total pancreatectomy was associated with a higher rate of clinically relevant complications compared to pancreatoduodenectomy and distal pancreatectomy (P = 0.004, Cramer’s V = 0.342, moderate correlation strength). The above-mentioned correlations are shown in Table 3.
Patients assessed as high risk for pancreatic fistula occurrence according to FRS have a statistically significant higher risk of postoperative complications in the form of clinically relevant pancreatic fistulas (P < 0.001, Cramer’s V = 0.467, moderate correlation strength). The high-risk FRS group is more likely to have clinically significant postoperative complications according to the Clavien-Dindo classification (P = 0.002, Phi = 0.352, moderate strength of correlation).
In cases of pancreatoduodenectomies in the high-risk FRS group, clinically relevant pancreatic fistula occurred in 50% of cases (P < 0.001, Cramer’s V = 0.668, strong correlation strength). In distal pancreatectomies, pancreatic fistula type B or C occurred in 45.5% of patients in the high-risk group (P < 0.001, Cramer’s V = 0.419, moderate correlation strength).
All cases of type B or C pancreatic fistulas had clinically relevant postoperative complications according to the Clavien-Dindo classification (P < 0.001, Phi = 0.837, strong correlation strength).
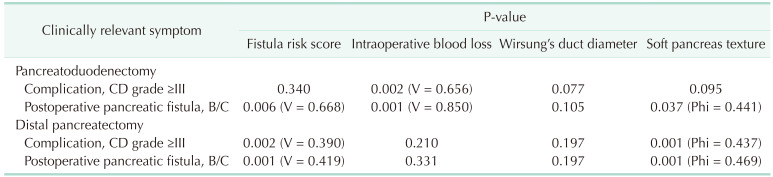
There are differences in the rate of influence of different risk factors on complications depending on the type of performed surgery. Intraoperative blood loss correlated with clinically relevant Clavien-Dindo grade (≥III) in pancreatoduodenectomies (P = 0.002, Cramer’s V = 0.656); however, it did not in distal pancreatectomies (P > 0.05). FRS and soft pancreas texture is correlated with Clavien-Dindo grade in distal pancreatectomies (P = 0.002, Cramer’s V = 0.390; P < 0.001, Phi = 0.437, respectively). Both FRS and soft pancreas texture was correlated with the manifestation of postoperative pancreatic fistula in pancreatoduodenectomies and distal pancreatectomies (P < 0.05). However, intraoperative blood loss influenced postoperative fistula in pancreatoduodenectomies only (P = 0.001, Cramer’s V = 0.850). The above-mentioned correlations are presented in Table 4.
Multivariate regression analysis was performed. Wirsung duct diameter was correlated with postoperative pancreatic fistula (odds ratio [OR], 0.261; 95% CI, 0.079–0.865; P = 0.028). Soft pancreas texture influenced complications on the Clavien-Dindo classification (OR, 4.854; 95% CI, 1.094–21.544; P = 0.038) (Table 5; Figs. 1, 2).
One of the most common complications in pancreatic surgery is the occurrence of postoperative pancreatic fistula [3]. In our study, clinically relevant pancreatic fistulas affected 19 operated patients (14.5%). The incidence of postoperative pancreatic fistulas is similar to data available in the literature, according to which they occur in 5%–30% of patients undergoing pancreatic resections for NETs, regardless of the type of surgery [2310]. Significant postoperative complications occurred in 30 cases (22.9%). The mortality rate after pancreatoduodenectomies and distal pancreatectomies in high-referral centers is up to 5% [1516]. The lowest mortality rate applies to enucleation procedures, where it is close to 0% [16]. In the study group, the mortality rate was 2.3%. Two deaths occurred after pancreatoduodenectomy, one after pancreatectomy, while no deaths were observed among patients after distal pancreatectomy.
Among the risk factors for postoperative pancreatic fistula is the texture of the pancreas described as soft [1718]. Postoperative fistulas are 2 times more common in cases of soft pancreas compared to firm texture, regardless of the histopathologic type of pancreatic tumor [219]. However, in cases of NETs compared to adenocarcinomas, there is no desmoplastic reaction in the pancreas [20]. This results in less frequent pancreatic fibrosis in NETs compared to other neoplasms and inflammatory lesions of the pancreas [21]. As a result, the soft pancreatic texture is more commonly found in NETs compared to adenocarcinomas [22]. Therefore, the histopathological diagnosis of NET is a risk factor for pancreatic fistula [21]. In our analysis, postoperative fistulas occurred in 37.5% of soft pancreases in comparison to 4.7% of pancreases with a firm texture. Multivariate analysis showed a high influence of soft pancreas texture on clinically relevant (type B or C) pancreatic fistula formation; however, results were not statistically significant (OR, 5.394; 95% CI, 0.954–30.489; P = 0.057). A soft pancreas is also a risk factor for significant postoperative complications determined by the Clavien-Dindo classification [23]. The percentage of patients who develop these complications is approximately 30% higher for pancreas texture defined as soft compared to firm [23]. Similar differences were found in our study where clinically relevant postoperative complications occurred in 41.9% of cases of soft texture and 13.6% of cases of firm pancreatic texture. Multivariate analysis confirmed these findings, soft pancreas texture correlates with significant (≥III) postoperative complications on Clavien-Dindo classification (OR, 4.854; 95% CI, 1.094–21.544; P = 0.038). An undeniable shortcoming of pancreatic texture assessment is that it is a subjective assessment made by the operating surgeon [17]. However, for an experienced surgeon (>400 of performed pancreatic resections), it is a recognized examination [1724].
Another risk factor for the occurrence of the pancreatic fistula is the diameter of the Wirsung duct [24]. A noticeable increase in the incidence of pancreatic fistulas is found when the Wirsung duct diameter is ≤3 mm [24]. In cases with pancreatic duct diameter ≤3 mm, postoperative pancreatic fistulas occur 3 times more often than when their diameter is >3 mm [10]. Martin et al. [25] reported that the diameter of the Wirsung duct affects the incidence of pancreatic fistulas in pancreatoduodenectomies, but has no effect on the incidence of fistulas after distal pancreatectomies. In our study, all (100%) clinically significant pancreatic fistulas occurred in cases with a Wirsung duct diameter of ≤3 mm. The correlation of the incidence of pancreatic fistulas with Wirsung duct diameter was a statistically significant negative correlation (as the measured diameter decreases, the incidence of type B and C pancreatic fistulas increases). Multivariate analysis showed a significant correlation between Wirsung duct diameter and clinically relevant postoperative fistula (B and C). As the duct’s diameter gets smaller, there is a higher risk of fistula formation (OR, 0.261; 95% CI, 0.079–0.865, P = 0.028). In distal pancreatic resections, complications are often due to the difficulty of visualizing the Wirsung duct and lack of safety of its closure and, in the case of pancreatoduodenectomy, from the difficulty of anastomosing the small pancreatic duct with the intestine [25]. Wirsung duct dilatation is approximately 8 times more common in adenocarcinomas compared to NETs, regardless of the location of the lesion in the pancreas [26]. This is another factor contributing to the higher incidence of pancreatic fistulas in NETs.
In the reviewed literature, there are predominant reports that the Wirsung duct diameter of ≤3 mm is related to the degree of postoperative complications [1827]. According to Jiwani and Chawla [15], similar to our analysis, the diameter of the pancreatic duct does not correlate with the degree of complications defined by the Clavien-Dindo classification. Multivariate binary regression analysis also corresponded to univariate analysis in this regard (P > 0.05).
Risk factors for pancreatic fistula also include intraoperative blood loss [23]. Patients with intraoperative blood loss of more than 1,000 mL are at high risk of this complication [23]. According to the authors of the FRS scale, blood loss of more than 700 mL during surgery is considered significant [10]. The groups of patients with pancreatic fistulas and clinically relevant postoperative complications have higher intraoperative blood loss compared to patients without complications. In our analysis, no patients had blood loss of more than 1,000 mL. We found a more than 5-fold higher incidence of pancreatic fistulas in patients who had intraoperative blood loss of >700 mL compared to blood loss of ≤700 mL. This group of patients also had a 4-fold higher incidence of significant clinical complications. Intraoperative blood loss is a risk factor also associated with the extent of surgery. In our analysis, the highest blood loss was in patients who underwent total pancreatectomy.
According to the literature, pancreatic fistulas are about 2 times more common in distal pancreatectomies compared to pancreatoduodenectomies [1928]. Similar differences in the incidence of postoperative fistulas were observed in our study group, but the difference was not statistically significant. Moreover, as shown in our analysis, clinically relevant pancreatic fistula also occurs in cases of enucleation. In resections of the head of pancreas compared to distal pancreatectomies, clinically significant complications are 10%–15% more frequently associated; also in cases of resection of NETs [1928]. In our analysis, significant postoperative complications affected 25.8% of patients after pancreatoduodenectomy and 20.5% of patients after distal pancreatic resections. There are differences in the rate of influence of different risk factors on complications depending on the type of performed surgery. According to our study, in pancreatoduodenectomies, intraoperative blood loss influences complications on the Clavien-Dindo classification and postoperative pancreatic fistula formation, while soft pancreatic texture and FRS is correlated with pancreatic fistulas only (P < 0.05 in all cases). However, in distal pancreatectomies, FRS and soft pancreatic texture correlates with postoperative pancreatic fistulas and complications on the Clavien-Dindo classification (P < 0.05).
The FRS scale is a tool used to assess the risk of occurrence of postoperative pancreatic fistula [29]. According to the literature, the incidence of the clinically significant pancreatic fistula is approximately 6.5% for the low-risk group, 13% for the moderate-risk group, and over 30% for the high-risk group [11]. There is a small amount of literature available that has analyzed the use of this scale after resections of pancreatic NETs. In our analysis of pancreatic NETs, fistulas did not occur in any patients in the low-risk group. In the study population, 6.5% of patients in the moderate-risk group and 46.4% in the high-risk group were affected by pancreatic fistula. Both pancreatoduodenectomy and distal pancreatectomy patients displayed significant correlations between the FRS score and the occurrence of pancreatic fistula. The correlation was stronger in the pancreatoduodenectomy group than in the distal pancreatectomy cohort. An undeniable limitation of the use of the FRS scale for pancreatic NETs is that the diameter of the Wirsung duct, which is impossible to measure in the case of enucleations, is used for risk assessment.
Moreover, the performed analysis also demonstrated an association between the FRS scale and the occurrence of significant postoperative complications after resections of NETs. Clavien-Dindo grade III and IV complications were 3 times more common in the high-risk pancreatic fistula group compared to the low and moderate-risk groups. The observed association of the occurrence of significant postoperative complications on the type of fistula will also allow, with a combination of the FRS scale, a prediction of possible complications of grade III and higher on the Clavien-Dindo classification. In the analyzed group, pancreatic fistula type B or C was found in all cases of postoperative complications of III–V grade according to the Clavien-Dindo classification. The use of this scale in assessing the severity of postoperative complications seems important because clinically significant pancreatic fistulas, especially type C, are associated with a high risk of reoperations [30].
There are limitations to this study. First of all, FRS cannot be used in all types of resections, because not all of them visualize the pancreatic duct for measurement. Furthermore, it is a retrospective study restricted to 131 patients. However, the sample size is rather small because of the rarity of these tumors.
Soft pancreatic texture, intraoperative blood loss of >700 mL, and small Wirsung duct are risk factors for clinically significant postoperative pancreatic fistula after resection of pancreatic NET. Among the risk factors analyzed, only the diameter of the Wirsung duct is not associated with the rate of postoperative Clavien-Dindo complications. Pancreatic fistulas were more frequently found in distal pancreatectomies in comparison with pancreatoduodenectomies. Significant postoperative complications are most common after total pancreatectomy compared to other pancreatic resections. FRS may be applied not only in pancreatoduodenectomies but also in distal pancreatectomies.
The FRS scale is crucial not only in assessing the risk of pancreatic fistula but also in significant postoperative complications after pancreatoduodenectomies and distal pancreatectomies for pancreatic NETs. The occurrence of clinically significant pancreatic fistula affects the degree of postoperative complications determined by the Clavien-Dindo classification.
References
1. Pedrazzoli S. Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): a systematic review and analysis of the POPF-related mortality rate in 60,739 patients retrieved from the English literature published between 1990 and 2015. Medicine (Baltimore). 2017; 96:e6858. PMID: 28489778.
2. Ecker BL, McMillan MT, Allegrini V, Bassi C, Beane JD, Beckman RM, et al. Risk factors and mitigation strategies for pancreatic fistula after distal pancreatectomy: analysis of 2026 resections from the international, multi-institutional distal pancreatectomy study group. Ann Surg. 2019; 269:143–149. PMID: 28857813.
3. Unegbu FC, Anjum F. Pancreatic Fistula. Mutignani M, Albert JG, Fabbri C, editors. Endotherapy in biliopancreatic diseases: ERCP meets EUS: two techniques for one vision. Springer Cham;2020. p. 551–560.
4. Jilesen AP, van Eijck CH, in’t Hof KH, van Dieren S, Gouma DJ, van Dijkum EJ. Postoperative complications, in-hospital mortality and 5-year survival after surgical resection for patients with a pancreatic neuroendocrine tumor: a systematic review. World J Surg. 2016; 40:729–748. PMID: 26661846.
5. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017; 161:584–591. PMID: 28040257.
6. Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016; 103:153–171. PMID: 26742109.
7. Albers MB, Almquist M, Bergenfelz A, Nordenström E. Complications of surgery for gastro-entero-pancreatic neuroendocrine neoplasias. Langenbecks Arch Surg. 2020; 405:137–143. PMID: 32291468.
8. Guo CX, Shen YN, Zhang Q, Zhang XZ, Wang JL, Gao SL, et al. Prediction of postoperative pancreatic fistula using a nomogram based on the updated definition. Ann Surg Treat Res. 2020; 98:72–81. PMID: 32051815.
9. Tessman D, Chou J, Shebrain S, Munene G. Surgical outcomes of distal pancreatectomy in elderly patients. Am Surg. 2022; 88:115–119. PMID: 33342301.
10. Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013; 216:1–14. PMID: 23122535.
11. Miller BC, Christein JD, Behrman SW, Drebin JA, Pratt WB, Callery MP, et al. A multi-institutional external validation of the fistula risk score for pancreatoduodenectomy. J Gastrointest Surg. 2014; 18:172–179. discussion 179-80. PMID: 24002771.
12. Kos-Kudła B, Rosiek V, Borowska M, Bałdys-Waligórska A, Bednarczuk T, Blicharz-Dorniak J, et al. Pancreatic neuroendocrine neoplasms - management guidelines (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol Pol. 2017; 68:169–197. PMID: 28540973.
13. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009; 250:187–196. PMID: 19638912.
14. Zovak M, Mužina Mišić D, Glavčić G. Pancreatic surgery: evolution and current tailored approach. Hepatobiliary Surg Nutr. 2014; 3:247–258. PMID: 25392836.
15. Jiwani A, Chawla T. Risk factors of pancreatic fistula in distal pancreatectomy patients. Surg Res Pract. 2019; 2019:4940508. PMID: 31396547.
16. Stella M, Bissolati M, Gentile D, Arriciati A. Impact of surgical experience on management and outcome of pancreatic surgery performed in high- and low-volume centers. Updates Surg. 2017; 69:351–358. PMID: 28215039.
17. Cao X, Zhu S, Luo G, Huang G. Soft pancreas should be assessed histopathologically for fibrosis to predict postoperative pancreatic fistula after pancreaticoduodenectomy. Asian J Surg. 2021; 44:421–422. PMID: 33246802.
18. Petrova E, Lapshyn H, Bausch D, D’Haese J, Werner J, Klier T, et al. Risk stratification for postoperative pancreatic fistula using the pancreatic surgery registry StuDoQ|Pancreas of the German Society for General and Visceral Surgery. Pancreatology. 2019; 19:17–25. PMID: 30563791.
19. Kumar S, Chandra A, Madhavan SM, Kumar D, Chauhan S, Pandey A, et al. Predictors and outcomes of pancreatic fistula following pancreaticoduodenectomy: a dual center experience. Indian J Surg Oncol. 2021; 12:22–30. PMID: 33814828.
20. Schober M, Jesenofsky R, Faissner R, Weidenauer C, Hagmann W, Michl P, et al. Desmoplasia and chemoresistance in pancreatic cancer. Cancers (Basel). 2014; 6:2137–2154. PMID: 25337831.
21. Fendrich V, Waldmann J, Bartsch DK, Langer P. Surgical management of pancreatic endocrine tumors. Nat Rev Clin Oncol. 2009; 6:419–428. PMID: 19506584.
22. Bartolini I, Bencini L, Risaliti M, Ringressi MN, Moraldi L, Taddei A. Current management of pancreatic neuroendocrine tumors: from demolitive surgery to observation. Gastroenterol Res Pract. 2018; 2018:9647247. PMID: 30140282.
23. Satoi S, Yamamoto T, Motoi F, Matsumoto I, Yoshitomi H, Amano R, et al. Clinical impact of developing better practices at the institutional level on surgical outcomes after distal pancreatectomy in 1515 patients: domestic audit of the Japanese Society of Pancreatic Surgery. Ann Gastroenterol Surg. 2018; 2:212–219. PMID: 29863185.
24. Eshmuminov D, Schneider MA, Tschuor C, Raptis DA, Kambakamba P, Muller X, et al. Systematic review and meta-analysis of postoperative pancreatic fistula rates using the updated 2016 International Study Group pancreatic fistula definition in patients undergoing pancreatic resection with soft and hard pancreatic texture. HPB (Oxford). 2018; 20:992–1003. PMID: 29807807.
25. Martin AN, Narayanan S, Turrentine FE, Bauer TW, Adams RB, Zaydfudim VM. Pancreatic duct size and gland texture are associated with pancreatic fistula after pancreaticoduodenectomy but not after distal pancreatectomy. PLoS One. 2018; 13:e0203841. PMID: 30212577.
26. Baxi AC, Jiang Q, Hao J, Yang Z, Woods K, Keilin S, et al. The effect of solid pancreatic mass lesions on pancreatic duct diameter at endoscopic ultrasound. Endosc Ultrasound. 2017; 6:103–108. PMID: 28440235.
27. Jilesen AP, van Eijck CH, Busch OR, van Gulik TM, Gouma DJ, van Dijkum EJ. Postoperative outcomes of enucleation and standard resections in patients with a pancreatic neuroendocrine tumor. World J Surg. 2016; 40:715–728. PMID: 26608956.
28. Valente R, Lykoudis P, Tamburrino D, Inama M, Passas I, Toumpanakis C, et al. Major postoperative complications after pancreatic resection for P-NETS are not associated to earlier recurrence. Eur J Surg Oncol. 2017; 43:2119–2128. PMID: 28821361.
29. Mohamed A, Nicolais L, Fitzgerald TL. Revisiting the pancreatic fistula risk score: clinical nomogram accurately assesses risk. Am Surg. 2021; 31348211047471. PMID: 34633224.
30. Chong E, Ratnayake B, Lee S, French JJ, Wilson C, Roberts KJ, et al. Systematic review and meta-analysis of risk factors of postoperative pancreatic fistula after distal pancreatectomy in the era of 2016 International Study Group pancreatic fistula definition. HPB (Oxford). 2021; 23:1139–1151. PMID: 33820687.
Fig. 1
Forest plot regarding multivariate binary regression analysis of the influence of different risk factors on the manifestation of postoperative pancreatic fistula. OR, odds ratio; CI, confidence interval.
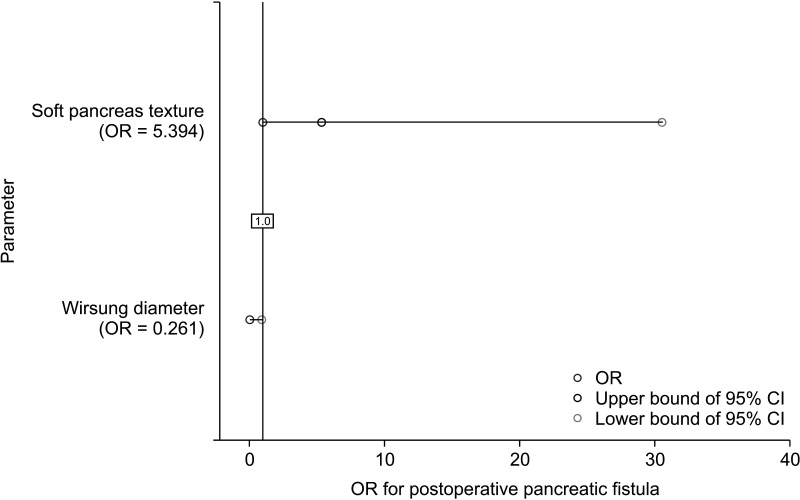
Fig. 2
Forest plot regarding multivariate binary regression analysis of the influence of different risk factors on complications according to Clavien-Dindo classification after performed surgery. OR, odds ratio; CI, confidence interval.
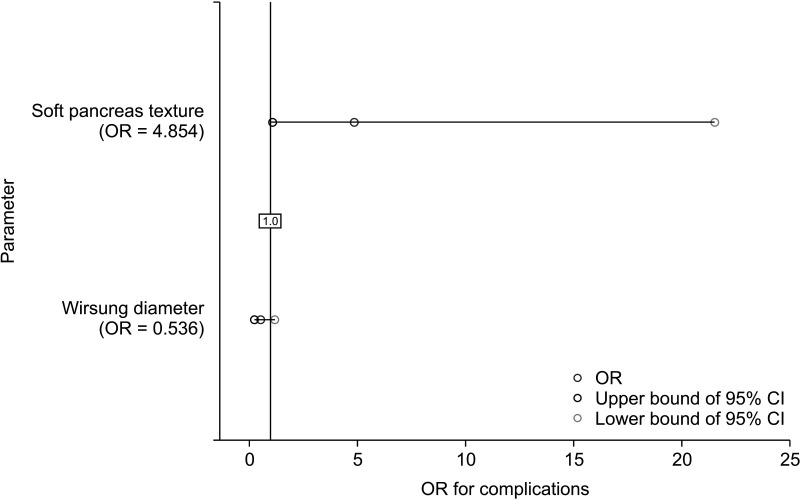
Table 2
Univariate correlation analysis of different risk factors, fistula risk score, and type of performed surgery on manifestation of postoperative pancreatic fistula
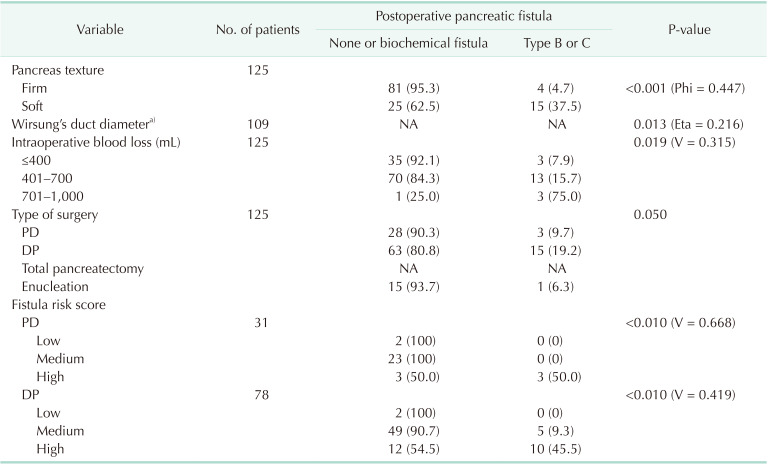
Table 3
Univariate correlation analysis of different risk factors and types of performed surgery on manifestation of postoperative complications




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download