Abstract
Purpose
This study aimed to identify prognostic factors based on treatment outcomes for congenital diaphragmatic hernia (CDH) at a single-center and to identify factors that may improve these outcomes.
Methods
Thirty-five neonates diagnosed with CDH between January 2011 and December 2021 were retrospectively analyzed. Pre- and postnatal factors were correlated and analyzed with postnatal clinical outcomes to determine the prognostic factors. Highest oxygenation index (OI) within 24 hours of birth was also calculated. Treatment strategy and outcome analysis of published literatures were also performed.
Results
Overall survival rate of this cohort was 60%. Four patients were unable to undergo anesthesia and/or surgery. Three patients who commenced extracorporeal membrane oxygenation (ECMO) post-surgery were non-survivors. Compared to the survivor group, the non-survivor group had a significantly higher occurrence of pneumothorax on the first day, need for high-frequency ventilator and inhaled nitric oxide use, and high OI within the first 24 hours. The non-survivor group showed an early trend towards the surgery timing and a greater number of patch closures. Area under the receiver operating characteristic curve was 0.878 with a sensitivity of 76.2% and specificity of 92.9% at an OI cutoff value of 7.75.
Congenital diaphragmatic hernia (CDH) is a rare form of congenital disease with a frequency of 1 in 2,000 to 5,000 births; however, CDH mortality is high [1-5]. Kim et al. [6. reported that CDH is the most frequent cause of death among infants aged <1 year (1.31%) between 2010 and 2013. Death of CDH patients is not due to the absence of the diaphragm itself, but due to secondary conditions, including pulmonary hypoplasia and pulmonary hypertension caused by infiltration of abdominal organs into the thoracic cavity due to the absence of the diaphragm.
In most patients with CDH, although variable in their severity, pulmonary hypoplasia and pulmonary hypertension are predictable, and the basic principle is the stabilization of vital signs and subsequent surgical correction of the CDH. Nevertheless, there are various proposed treatment guidelines, including those of the American Heart Association and American Thoracic Society [7], CDH EURO Consortium Group [8], and Canadian Congenital Diaphragmatic Hernia Collaborative [9], which still have disagreements in certain domains, although each institution applies a different treatment protocol. With advances in prenatal diagnosis and neonatal management, the survival rate of patients with CDH has shown an increasing trend. However, because of the variation in approaches adopted at each center regarding prenatal diagnosis and postnatal treatment, the reported rate of survival ranges substantially from 32% to 90% [10,11].
CDH is a disease with a high risk of mortality, and knowledge of its prognostic factors is crucial in the setting of treatment strategies, as well as for consultations with patients’ guardians. To date, various prognostic factors suggested include gestational age (GA) at prenatal diagnosis, whether the CDH is right-sided, associated chromosomal and/or major heart anomalies, lungto- head ratio (LHR), and observed-to-expected LHR. Thus, this study aimed to identify prognostic factors based on treatment outcomes of the past 11 years at a single-center, analyze these outcomes, and determine points of improvement in diagnostic and therapeutic approaches compared with those used in previous studies.
Medical records of neonates diagnosed with CDH at the neonatal intensive care unit (NICU) at the center between January 2011 and December 2021 were retrospectively analyzed. Participants included inborn and outborn neonates who were transferred to the center within 24 hours of birth. Patients with complex heart defects were excluded from the study.
The following demographic data and clinical characteristics were collected: GA at birth, birth weight, sex, mode of delivery, twin birth, small for GA, Apgar scores at 1 and 5 minutes, outborn, first day of pneumothorax, age at CDH repair, duration of assisted ventilation, duration of inhaled nitric oxide (iNO), duration of hospitalization, and outcome (survival). Prenatal and postnatal factors were investigated to identify poor prognostic factors related to mortality. Regarding the prenatal factors, GA at CDH diagnosis, right-sided CDH, liver herniation, and maternal factors, such as polyhydramnios, maternal age, maternal hypertension, and gestational diabetes mellitus were used. Highest oxygenation index (OI; fraction of inspired O2 [FiO2]×mean airway pressure [MAP]×100/partial pressure of oxygen in arterial blood), high-frequency oscillation (HFO), iNO within 24 hours of birth, herniated organs found in the thoracic cavity during surgery, as well as the highest OI, HFO, and iNO within 24 hours of operation, and the use of extracorporeal membrane oxygenation (ECMO) were considered postnatal factors. The MAP in the calculation was the set MAP in a case of HFO ventilation, or the calculated MAP using the following equation: PEEP+[(PIP–PEEP)×(ti/ti+te)] (PEEP, positive expiratory pressure; PIP, peak inspiratory pressure; ti, inspiratory time; te, expiratory time), in a case of conventional ventilator mode [12].
We maintained similar diagnostic and surgical methods during the study; there was no change in that preoperative stabilization was mainly performed with the current standard management that did not include the ECMO. The postnatal management provided to patients with CDH at the center was as follows: in cases of suspected prenatal CDH, the obstetrician, neonatologist, and surgeon made preparations prior to delivery. A neonatologist participated in all the deliveries. Immediately after the delivery, the neonate was transferred to the NICU for mechanical ventilation. In most cases, the conventional ventilation mode was first applied, and the mode was then switched to HFO ventilation in the absence of effective gas exchange. Echocardiography was performed within 24 hours of birth to detect pulmonary hypertension or structural abnormalities of the heart. We administered iNO therapy to infants with pulmonary hypertension when they had evidence of extrapulmonary right-to-left shunting and if the OI was greater than 25 despite effective ventilator management [13].
Immediate surgery within 48 hours of birth was the preferred choice at this center. The operation was performed by surgeons in the NICU, following the decision of the neonatologist based on the vital signs and results of radiography and echocardiography. Preoperative ECMO was avoided, considering the risk of potential hemorrhage and death during surgery. Postoperative ECMO indications were as follows: (1) OI >40 for ≥4 hours; (2) right and/or left ventricular dysfunction; (3) pressor-resistant hypotension; and (4) refractory acidosis and shock (pH <7 for 2 to 4 hours). The ECMO was not performed in the following cases: (1) irreversible brain damage; (2) lethal chromosome disorder; (3) uncontrolled bleeding or coagulopathy; (4) grade ≥3 intraventricular hemorrhage; (5) GA <34 weeks; and (6) weight <2 kg [14].
All statistical analyses were performed using R version 4.1.3 (R Core Team 2022, R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/). Comparisons between the two groups were evaluated using an independent 2-sample t-test. The Pearson’s chi-square test was used to analyze differences in the proportions of more than two categories. Logistic regression was used to detect significant covariates in the outcomes. Area under the receiver operating characteristic (ROC) curve (AUC) analysis was performed, and the optimal cutoff value of the preoperative OI in 24 hours after birth was evaluated based on the ROC.
During the study period, 38 patients with CDH were admitted into the NICU, of whom 35 were selected as the study population after excluding three patients with tetralogy of Fallot. The mean GA was 37.9±1.5 weeks, and the mean birth weight was 2,987.7±464.5 g. Twenty (57.1%) patients were males. Twenty-one (60%) patients needed iNO treatment for pulmonary hypertension, and the mean iNO treatment period was 5.6±5.2 days. Four patients were unable to undergo surgery because their clinical condition was so severe that they could not tolerate anesthesia and/or surgery. Twenty-one of the 35 patients survived with a 60% survival rate (Table 1). The mean number of hospitalization days for survivors was 27±18.3 and 6.1±6.8 days for non-survivors.
In the analysis of prenatal factors for prognosis prediction, no factor exhibited a significant between-group difference, including GA at diagnosis, right-sided CDH, and liver herniation (Table 2). On analysis of postnatal factors, compared with the survivor group, the non-survivor group had a significantly higher occurrence on the first day of pneumothorax (6 [42.9%] vs. 0, P= 0.002) and need for high-frequency ventilator (9 [64.3%] vs. 5 [23.8%], P=0.041) and iNO (11 [78.6%] vs. 4 [19%], P=0.001). The highest OI within 24 hours of birth was also significantly higher in the non-survivor group (32.4±22.7 vs. 7.7±9.5, P=0.001). Regarding the surgery, the non-survivor group showed an earlier trend in timing of the surgery (1.3±0.5 days vs. 2.1±1.1 days, P=0.015) and a greater number of patch closures (3 [30%] vs. 0, P=0.027). After the surgery, the highest OI within 24 hours of surgery (41.5±46.8 vs. 5.6±7.3, P=0.039) and need for high-frequency ventilation (8 [80%] vs. 6 [28.6%], P=0.018) and iNO (8 [80%] vs. 6 [28.6%], P=0.021) were also significantly higher and more frequent in the non-survivor group (Table 3). However, a multivariate analysis incorporating all the above factors did not identify a significant risk factor.
Four of the non-survivors could not be stabilized by conservative treatment after birth of which they eventually died without corrective surgery. Time of death was postnatal day 1 (n=1), postnatal day 2 (n=2), and postnatal day 4 (n=1), with an average OI of 53.1±15.6 on the first day of life. All the three patients who commenced ECMO post-surgery were non-survivors; they showed stable vital signs on the first day of life (mean preoperative OI, 7.8±3.2) and underwent corrective surgery, but they subsequently deteriorated. The ECMO was initiated after the surgery on days 3, 6, and 8 (mean OI, 65.9±23.6; just before the ECMO), but eventually, two patients died on postnatal day 20, and one died on postnatal day 15. The average ECMO period was 9.3±3.1 days.
The ROC curve analysis of the preoperative OI within 24 hours of birth was performed to identify an appropriate cutoff value predictive of mortality (Figure 1). The AUC was 0.878 with a sensitivity of 76.2% and a specificity of 92.9% at a cutoff value of 7.75.
In this study, the survival rate of the patients with CDH was 60%. Recent studies on CDH conducted by other centers in South Korea have also reported a similar survival rate of 57.6% [15] and 63.2% [16]. However, studies have reported a trend of survival improvement in CDH patients abroad, including 75.4% of CDH survival rates in Japan between 2006 and 2010 [17]. Considering that the pulmonary hypertension in CDH is a major risk factor for mortality, the stabilization strategy before and after the surgery is key to the difference in outcome. Many studies have reported improvements in survival after the introduction of ECMO. Morini et al. [18]. reported that the mortality rate decreased from 83.5% to 38.3% after the introduction of ECMO in patients with CDH. A simple survival rate comparison is not desirable, considering that each patient's risk factors, such as defect size and location are different. However, it is important to analyze institutional treatment outcomes and recent treatment trends in other institutions to improve patient outcomes.
Widely acknowledged mortality-related prenatal risk factors, such as GA at diagnosis, right-sided CDH, and liver herniation were not significant factors in this study. However, considering the small sample size of this study and unknown liver herniation in the four preoperative deaths, the trend reported by previous studies cannot be refuted. Notably, among five of the 35 (14.3%) patients with confirmed right-sided CDH, three survived, which was similar to the 66.7% survival rate reported by Jeong et al. [19]. Also, as survival was observed in a case of early diagnosis at 20 weeks, a poor prenatal prognostic factor should not be considered to predict death or be a reason to delay treatment. Importantly, as there is a limitation to the prediction of actual cardiopulmonary function based only on assessing anatomical structures, it is important to analyze other postnatal prognostic factors that may enable accurate prognostication. In this study, the percentage of outpatients transferred to the present center after birth at a different center, owing to lack of CDH detection during prenatal examinations was 20%. This indicates that despite advances in prenatal diagnosis, there are still neglected areas that need improvement. Thus, the analysis of postnatal prognostic factors is critical for establishing treatment plans and providing explanations to the patients’ guardians.
Pneumothorax has been shown to be a significant postnatal risk factor. Despite gentle conventional ventilator management through the use of low PEEP, PIP, and MAP in these patients, optimization of mechanical ventilation should still be the primary aim. Patch closure was shown to be a significant prognostic predictor, as it indicated a large defect. This is likely to have been associated with poor cardiopulmonary function (lung hypoplasia and pulmonary hypertension), owing to a large defect, rather than the surgical method of patch closure itself, which is consistent with the findings of Brandt et al. [20]. In contrast, OI reflects actual pulmonary function, and the optimal cutoff for OI in this study was 7.75. This was slightly lower than that reported in previous studies; a cutoff point of 18 with an AUC of 0.82 was observed in the study by Salas et al. [21]. A cutoff value of 40 with an AUC of 0.88 was observed in the study by Ruttenstock et al. [22], and the best OI set of 11 mm Hg was observed in the study by Oh et al. [16]. Although the variation may be due to differences in basic ventilator management, it seems necessary to consider other treatments applied simultaneously alongside the ventilator. It is likely challenging to identify the cause of such variations. In this study, it is apparent that in line with previous studies, the OI within 24 hours of birth was the most significant predictor of mortality and survival, with an AUC of 0.878, sensitivity of 0.762, and specificity of 0.929 when the optimal cutoff (threshold) was set to 7.75. In patients with CDH, arterial catheter insertion for 1 or more days during the early days after birth is anticipated to enable continuous blood pressure monitoring and blood gas testing. Considering this, OI is an indicator of hemodynamics, which fluctuates in real time and should be recognized for its value as a prognostic predictor, such that it may be used more actively in clinical practice.
Taken together, the mean timing of surgery for the patients was 1.8±1.0 days, which tended to be earlier than that of an institution with 84.6% survival rate (timing of surgery: survivors, 9.1±26.6 days; and non-survivors, 10.1±9 days) [20]. Furthermore, preoperative OI was found to be higher in the non-survivor group than in the survivor group, leading to earlier timing of surgery in this group. This was because it is believed that surgery was a necessity in those patients where stabilization could not be achieved by using only the ventilator, iNO, and drug treatment. As previously mentioned, if possible, the application of ECMO was avoided at the present center, owing to concerns regarding the invasive nature of ECMO and possible complications, such as hemorrhage. Our treatment strategy was based on the fact that the utility of ECMO is controversial in patients with CDH [8,23]. Analyzing subsequent progress, HFO and iNO use after surgery transiently increased in the survivor and non-survivor groups, which could be due to the fluctuation in hemodynamics caused by the invasive nature of the surgery. In contrast, the postoperative OI decreased in the survivor group, but increased in the non-survivor group. This suggests that while surgical correction may support further stabilization of cardiopulmonary function in CDH patients with stable hemodynamics, deciding on surgical correction too rapidly may not guarantee survival in CDH patients with signs of unstable hemodynamics in the presence of severe pulmonary hypoplasia and hypertension. Therefore, ECMO can only be considered for stabilization before surgery in patients with pulmonary hypertension who do not respond to conservative treatment. Additionally, all the three patients who underwent ECMO after the surgery died, which may have been a selection bias due to the fact that the most serious patients were likely to have been assessed as requiring ECMO. However, considering that the OI immediately before the ECMO commencement was already high, one could speculate that commencing the ECMO earlier may have resulted in better outcomes.
Looking at the treatment trends of other institutions in Korea and abroad, there are reports that the survival rate has been improved by actively attempting ECMO if necessary, with the principle of "post-stabilization surgery" rather than "fast corrective surgery." A report by Choi et al. [24] in Korea showed that the survival rate of patients with CDH undergoing ECMO was 31.4%, which is suboptimal, but shows a possibility of survival. Although the frequency of ECMO use varies between 11% to 61% among hospitals [25], Seetharamaiah et al. [26] reported that 34% (1,063/3,100) of patients with CDH were managed by ECMO support between 1995 and 2004. ECMO is not only widely used in newborns with CDH [27-29], but the application of ECMO has also been shown to increase the survival rate of patients who do not respond to conventional treatment [30,31]. Brandt et al. [20] introduced ECMO in 32.3% (n=21) of critically unstable CDH patients, of whom 66.7% survived. Hung et al. [32] reported that the survival rate of all patients with CDH was 84.6%, indicating a 57.1% survival rate within their ECMO group, which was a significant improvement compared to their outcomes prior to ECMO introduction. However, even if ECMO was used, disagreements regarding the timing of surgery remain. There are conflicting reports of early repair (within 72 hours on ECMO) [32,33] and late repair (after ECMO decannulation) [34,35], regarding the optimal surgical timing that increases the survival of CHD and decreases surgical morbidity; this requires further research.
This study has several limitations. As this was a single-center study, the sample size was small. Additionally, since this was a retrospective study, there was a limit to the analysis and interpretation because of missing data. The LHR was not routinely assessed among prenatal ultrasound results; therefore, analysis was not possible. However, this study is valuable in the presentation of its data in an attempt to find ways to improve the prognosis of this group of patients. By analyzing the experience of CDH outcomes in a single institution in Korea and reviewing previous studies, useful information can be obtained for rare conditions that are managed in a small number of hospitals.
Despite advances in medicine, a proportion of cases of CDH remains fatal. If the management does not stabilize patients with CDH, it will be difficult to expect an improvement in the survival rate. Our data analysis confirms the usefulness of OI. As the results of studies showing the effectiveness of ECMO in patients with CDH are accumulating, it is expected that the application of ECMO, based on the OI monitoring, may help improve the opportunity for these patients to achieve corrective surgery, as well as improve their prognosis. Above all, it is essential that a well-designed protocol is used to conduct a multicenter study prospectively and on a greater number of participants to demonstrate improved outcomes and to refine a validated protocol.
Notes
Ethical statement
This study was approved by the Institutional Review Board of the Pusan National University Yangsan Hospital (IRB No. 05- 2022-025). The requirement for informed consent was waived by the board.
Author contributions
Conception or design: S.J., Y.M.H.
Acquisition, analysis, or interpretation of data: S.J., M.H.J., S.H.J., S.J.P., N.L., M.H.B., K.H.P., S.Y.B., S.H.K., Y.H.C., C.K., Y.M.H.
Drafting the work or revising: S.J., Y.M.H.
Final approval of the manuscript: All authors read and approved the final manuscript.
REFERENCES
1. Tsao K, Lally KP. The Congenital Diaphragmatic Hernia Study Group: a voluntary international registry. Semin Pediatr Surg. 2008; 17:90–7.
2. Logan JW, Cotten CM, Goldberg RN, Clark RH. Mechanical ventilation strategies in the management of congenital diaphragmatic hernia. Semin Pediatr Surg. 2007; 16:115–25.
3. Losty PD. Congenital diaphragmatic hernia: where and what is the evidence? Semin Pediatr Surg. 2014; 23:278–82.
4. Bohn D. Congenital diaphragmatic hernia. Am J Respir Crit Care Med. 2002; 166:911–5.
5. Downard CD, Jaksic T, Garza JJ, Dzakovic A, Nemes L, Jennings RW, et al. Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg. 2003; 38:729–32.
6. Kim SB, Jang MJ, Song YH, Jung SY, Oh JS, Lim JW. Trends and characteristics of mortality associated with congenital anomalies in Korean children under 5 years of age. Neonatal Med. 2021; 28:99–107.
7. Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015; 132:2037–99.
8. Snoek KG, Reiss IK, Greenough A, Capolupo I, Urlesberger B, Wessel L, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus. 2015 Update. Neonatology. 2016; 110:66–74.
9. Canadian Congenital Diaphragmatic Hernia Collaborative, Puligandla PS, Skarsgard ED, Offringa M, Adatia I, Baird R, et al. Diagnosis and management of congenital diaphragmatic hernia: a clinical practice guideline. CMAJ. 2018; 190:E103–12.
10. Wynn J, Krishnan U, Aspelund G, Zhang Y, Duong J, Stolar CJ, et al. Outcomes of congenital diaphragmatic hernia in the modern era of management. J Pediatr. 2013; 163:114–9.
11. Cruz-Martinez R, Etchegaray A, Molina-Giraldo S, Nieto-Castro B, Gil Guevara E, Bustillos J, et al. A multicentre study to predict neonatal survival according to lung-to-head ratio and liver herniation in fetuses with left congenital diaphragmatic hernia (CDH): hidden mortality from the Latin American CDH Study Group Registry. Prenat Diagn. 2019; 39:519–26.
12. Glenski JA, Marsh HM, Hall RT. Calculation of mean airway pressure during mechanical ventilation in neonates. Crit Care Med. 1984; 12:642–4.
13. Weems MF, Jancelewicz T, Sandhu HS. Congenital diaphragmatic hernia: maximizing survival. NeoReviews. 2016; 17:e705–18.
14. Lima M, Reinberg O. Neonatal surgery: contemporary strategies from fetal life to the first year of age. Cham: Springer;2019. p. 17.
15. Ahn JH, Jung YH, Shin SH, Kim HY, Kim EK, Kim HS. Respiratory severity score as a predictive factor for the mortality of congenital diaphragmatic hernia. Neonatal Med. 2018; 25:102–8.
16. Oh C, Youn JK, Han JW, Yang HB, Lee S, Seo JM, et al. Predicting survival of congenital diaphragmatic hernia on the first day of life. World J Surg. 2019; 43:282–90.
17. Ukiyama E. Treatment for congenital diaphragmatic hernia: clinical guidelines. Pediatr Int. 2021; 63:369–70.
18. Morini F, Goldman A, Pierro A. Extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia: a systematic review of the evidence. Eur J Pediatr Surg. 2006; 16:385–91.
19. Jeong J, Lee BS, Cha T, Jung E, Kim EA, Kim KS, et al. Prenatal prognostic factors for isolated right congenital diaphragmatic hernia: a single center's experience. BMC Pediatr. 2021; 21:460.
20. Brandt JB, Werther T, Groth E, Kung E, Golej J, Berger A. Risk factors for mortality in infants with congenital diaphragmatic hernia: a single center experience. Wien Klin Wochenschr. 2021; 133:674–9.
21. Salas GL, Otano JC, Cannizzaro CM, Mazzucchelli MT, Goldsmit GS. Congenital diaphragmatic hernia: postnatal predictors of mortality. Arch Argent Pediatr. 2020; 118:173–9.
22. Ruttenstock E, Wright N, Barrena S, Krickhahn A, Castellani C, Desai AP, et al. Best oxygenation index on day 1: a reliable marker for outcome and survival in infants with congenital diaphragmatic hernia. Eur J Pediatr Surg. 2015; 25:3–8.
23. Kays DW. ECMO in CDH: is there a role? Semin Pediatr Surg. 2017; 26:166–70.
24. Choi W, Cho WC, Choi ES, Yun TJ, Park CS. Outcomes after extracorporeal membrane oxygenation in neonates with congenital diaphragmatic hernia: a single-center experience. J Chest Surg. 2021; 54:348–55.
25. Logan JW, Rice HE, Goldberg RN, Cotten CM. Congenital diaphragmatic hernia: a systematic review and summary of best-evidence practice strategies. J Perinatol. 2007; 27:535–49.
26. Seetharamaiah R, Younger JG, Bartlett RH, Hirschl RB; Congenital Diaphragmatic Hernia Study Group. Factors associated with survival in infants with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 2009; 44:1315–21.
27. Heiss K, Manning P, Oldham KT, Coran AG, Polley TZ Jr, Wesley JR, et al. Reversal of mortality for congenital diaphragmatic hernia with ECMO. Ann Surg. 1989; 209:225–30.
28. Lally KP, Paranka MS, Roden J, Georgeson KE, Wilson JM, Lillehei CW, et al. Congenital diaphragmatic hernia. Stabilization and repair on ECMO. Ann Surg. 1992; 216:569–73.
29. Reickert CA, Hirschl RB, Atkinson JB, Dudell G, Georgeson K, Glick P, et al. Congenital diaphragmatic hernia survival and use of extracorporeal life support at selected level III nurseries with multimodality support. Surgery. 1998; 123:305–10.
30. Hoffman SB, Massaro AN, Gingalewski C, Short BL. Predictors of survival in congenital diaphragmatic hernia patients requiring extracorporeal membrane oxygenation: CNMC 15-year experience. J Perinatol. 2010; 30:546–52.
31. Bryner BS, Kim AC, Khouri JS, Drongowski RA, Bruch SW, Hirschl RB, et al. Right-sided congenital diaphragmatic hernia: high utilization of extracorporeal membrane oxygenation and high survival. J Pediatr Surg. 2009; 44:883–7.
32. Hung WT, Huang SC, Mazloum DE, Lin WH, Yang HH, Chou HC, et al. Extracorporeal membrane oxygenation for neonatal congenital diaphragmatic hernia: the initial single-center experience in Taiwan. J Formos Med Assoc. 2017; 116:333–9.
33. Fallon SC, Cass DL, Olutoye OO, Zamora IJ, Lazar DA, Larimer EL, et al. Repair of congenital diaphragmatic hernias on extracorporeal membrane oxygenation (ECMO): does early repair improve patient survival? J Pediatr Surg. 2013; 48:1172–6.
34. Congenital Diaphragmatic Hernia Study Group, Bryner BS, West BT, Hirschl RB, Drongowski RA, Lally KP, et al. Congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: does timing of repair matter? J Pediatr Surg. 2009; 44:1165–71.
35. Partridge EA, Peranteau WH, Rintoul NE, Herkert LM, Flake AW, Adzick NS, et al. Timing of repair of congenital diaphragmatic hernia in patients supported by extracorporeal membrane oxygenation (ECMO). J Pediatr Surg. 2015; 50:260–2.
Figure 1.
Area under the receiver operating characteristic curve (AUC) of the highest preoperative oxygenation index within 24 hours of birth.
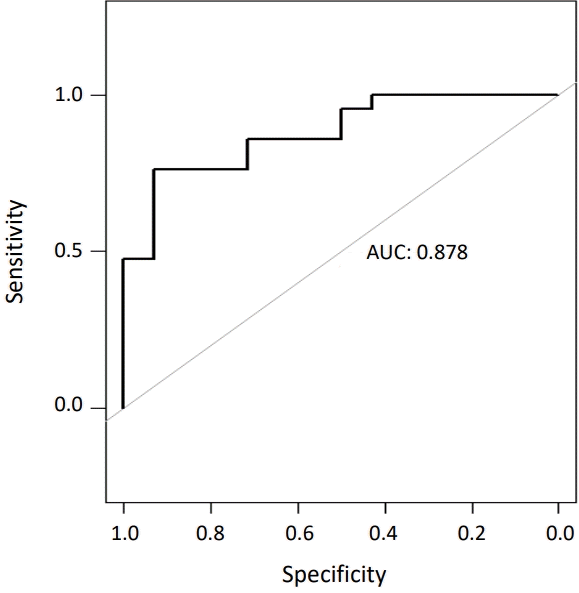
Table 1.
Baseline Characteristics of Study Object Infants with Congenital Diaphragmatic Hernia
Table 2.
Prenatal Risk Factors for Mortality of Congenital Diaphragmatic Hernia
| Variable | Survivors (n=21) | Non-survivors (n=14) | P-value |
|---|---|---|---|
| GA at CDH diagnosis (wk) | 28.2±6.0 | 25.6±3.9 | 0.178 |
| Site (right side) | 3 (14.3) | 2 (14.3) | 1.000 |
| Liver herniation | 4 (19) | 4 (40)* | 0.381 |
| Polyhydramnios† | 2 (11.8) | 4 (36.4) | 0.174 |
| Maternal age | 32.1±2.8 | 33.3±3.6 | 0.327 |
| Maternal hypertension | 0 | 1 (7.1) | 0.400 |
| Maternal GDM | 3 (14.3) | 0 | 0.259 |
Table 3.
Postnatal Clinical Outcome for the Mortality of Congenital Diaphragmatic Hernia
| Variable | Survival (n=21) | Non-survivor (n=14) | P-value |
|---|---|---|---|
| Gestational age (wk) | 38.1±1.2 | 37.7±1.9 | 0.445 |
| Birth weight (g) | 2,997.6±392.1 | 2,972.9±572.2 | 0.889 |
| SGA | 2 (9.5) | 2 (14.3) | 1.000 |
| Apgar score at 1 min | 5.2±1.9 | 3.7±2.3 | 0.061 |
| Apgar score at 5 min | 7.1±1.2 | 6.2±2.3 | 0.167 |
| Preoperative | |||
| Pneumothorax before OP (1st day) | 0 | 6 (42.9) | 0.002 |
| OI in 24 hr after birth | 7.7±9.5 | 32.4±22.7 | 0.001 |
| HFO applied | 5 (23.8) | 9 (64.3) | 0.041 |
| HFO (d) | 0.3±0.7 | 0.7±0.6 | 0.105 |
| iNO applied | 4 (19) | 11 (78.6) | 0.001 |
| iNO (d) | 0.3±0.7 | 0.9±0.5 | 0.011 |
| Age at surgery (postnatal d) | 2.1±1.1 | 1.3±0.5 | 0.015 |
| Surgical findings | |||
| Patch closure d/t large defect | 0 | 3 (30) | 0.027 |
| Hernia sac | 7 (33.3) | 2 (20) | 0.677 |
| Intrathoracic liver | 4 (19) | 4 (40) | 0.381 |
| Intrathoracic stomach | 11 (52.4) | 4 (40) | 0.704 |
| Intrathoracic bowel | 18 (85.7) | 8 (80) | 1.000 |
| Other intrathoracic solid organ | 12 (57.1) | 8 (80) | 0.262 |
| Postoperative* | |||
| OI after OP | 5.6±7.3 | 41.5±46.8 | 0.039 |
| HFO applied | 6 (28.6) | 8 (80) | 0.018 |
| HFO (d) | 1.6±3.3 | 4±5.0 | 0.193 |
| iNO applied | 6 (28.6) | 8 (80) | 0.021 |
| iNO (d) | 2.5±4.7 | 4.8±5.8 | 0.282 |
| ECMO applied | 0 | 3 (30) | 0.027 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download