Abstract
Neonatal thyrotoxicosis is rare and most of the cases are secondary to maternal Graves’ disease. It is usually transient, but can be associated with significant morbidity and mortality if not recognized promptly and treated adequately. Neonates born to mothers treated with antithyroid drugs or those who receive maternal thyroid blocking antibodies may exhibit normal thyroid function or even hypothyroidism at birth. Since there may not be any obvious symptoms of hyperthyroidism at birth, it may be overlooked. Therefore, such neonates should be evaluated properly and monitored regularly to prevent serious complications of hyperthyroidism. We report a case of a 21-day-old male infant who developed thyrotoxicosis with dyspnea, irritability, tachycardia, and cardiac insufficiency. He was born to a mother who was treated for Graves’ disease with antithyroid drugs during pregnancy. We have also discussed the importance of careful examination and monitoring to prevent the development of clinical hyperthyroidism.
Most of the cases of neonatal thyrotoxicosis are secondary to maternal Graves’ disease (GD). It is a very rare disease that can also occur secondary to activating mutations in the thyroid-stimulating hormone (TSH) receptor gene or McCune-Albright syndrome [1]. In addition, there have been reports of hyperthyroidism in newborns using topical iodide [2]. GD occurs in 0.1% to 0.4% of all pregnancies. Neonatal Graves’ disease (NGD) is observed in only 1% to 5% of the infants born to these mothers [1]. TSH receptor antibodies (TRAb) in mothers cross the placenta and stimulate the fetal thyroid gland, which can cause hyperthyroidism or thyrotoxicosis in the fetus or newborn. In mothers with GD, TRAb levels are highest in the first trimester of pregnancy and decrease as gestation progresses. Higher antibody levels in the mother during the third trimester are associated with a greater risk of GD in the fetus and newborn [3].
Maternal TSH receptor-stimulating antibodies (TSAb), thyroidblocking antibodies (TBAb), and maternal antithyroid drugs (ATDs) can pass through the placenta. The balance between TSAb and TBAb as well as maternal ATD dose influence the thyroid status of the fetus and neonate and the fluctuation of maternal antithyroid antibody titers may result in various risks to the fetus or neonate [1]. Since neonates born to mothers whose thyroid function is being controlled with ATDs may not have obvious symptoms of hyperthyroidism at birth, it may be overlooked [4].
Although NGD is rare and usually transient, it is associated with immediate and long-term morbidities. Cardiac insufficiency is a major risk among these infants and early detection and treatment are important to prevent severe complications [1,5,6].
We report a case of a 21-day-old male infant who developed thyrotoxicosis with dyspnea, irritability, tachycardia, and cardiac insufficiency. He was born to a mother who had been treated for GD with ATDs during pregnancy.
A 21-day-old male infant was referred by a local medical center for tachypnea, irritability, and poor sleep. He was delivered by repeated caesarean section at 39 weeks of gestation without any perinatal problems. He had a birth weight of 3,700 g. He was the second child in his family. His mother was 35 years of age and had no prior medical history. She was diagnosed with GD in the first trimester, started taking propylthiouracil (250 mg/day), and maintained normal thyroid levels until childbirth. She had no TRAb records. After birth, TSH and thyroxine (T4) levels of the infant were normal in the neonatal screening test. The infant and his mother stayed in the postpartum care center until the day before admission without undergoing a follow-up thyroid function test. The infant gained weight well, but had shortness of breath during or after feeding, which gradually aggravated.
On admission, he had tachypnea, chest wall retraction, and high appetite. He did not exhibit prominent goiter, staring appearance, or eyelid retraction. His body weight was 4.5 kg (25th to 50th percentile), height was 57.1 cm (90th to 95th percentile), and head circumference was 39 cm (>97th percentile). His blood pressure, pulse rate, respiratory rate, oxygen saturation, and body temperature were 90/40mm Hg, 150 beats/min, 60 breaths/min, 98%, and 37°C, respectively. Initial laboratory findings were as follows: hemoglobin, 14.3 g/dL; white blood cell count, 7,400/mm3; platelet count, 265,000/mm3; aspartate transaminase, 17 IU/L; alanine transaminase, 15 IU/L; pro-brain natriuretic peptide, 2,443 pg/mL (reference range, 9.2 to 83.9); and C-reactive protein, 1.1 mg/L (reference range, <5). Thyroid function test revealed elevated free thyroxine (FT4) (6.03 ng/dL; reference range, 0.9 to 2.3), elevated total triiodothyronine (T3) (3.23 ng/mL; reference range, 1.05 to 3.45), and decreased TSH (<0.1 μIU/mL; reference range, 0.5 to 6.5). TRAb level was 18.91 IU/L (reference range, <1.22). TSAb level was 492% (reference range, <140%). Tests for thyroid peroxidase and thyroglobulin antibodies were negative. Chest radiography revealed cardiomegaly, with a cardiothoracic ratio of 65% in the anteroposterior view (Figure 1). Echocardiography showed decreased left ventricular contractility (ejection fraction, 46.96%; fractional shortening, 21.79%). Ultrasound examination performed on day 8 of hospitalization revealed that both the thyroid lobes were enlarged with subtle coarse echogenicity (Figure 2A, B). A Tc-99m pertechnetate thyroid scan performed on day 9 of hospitalization revealed an increased uptake in both the thyroid lobes (Figure 2C) [7].
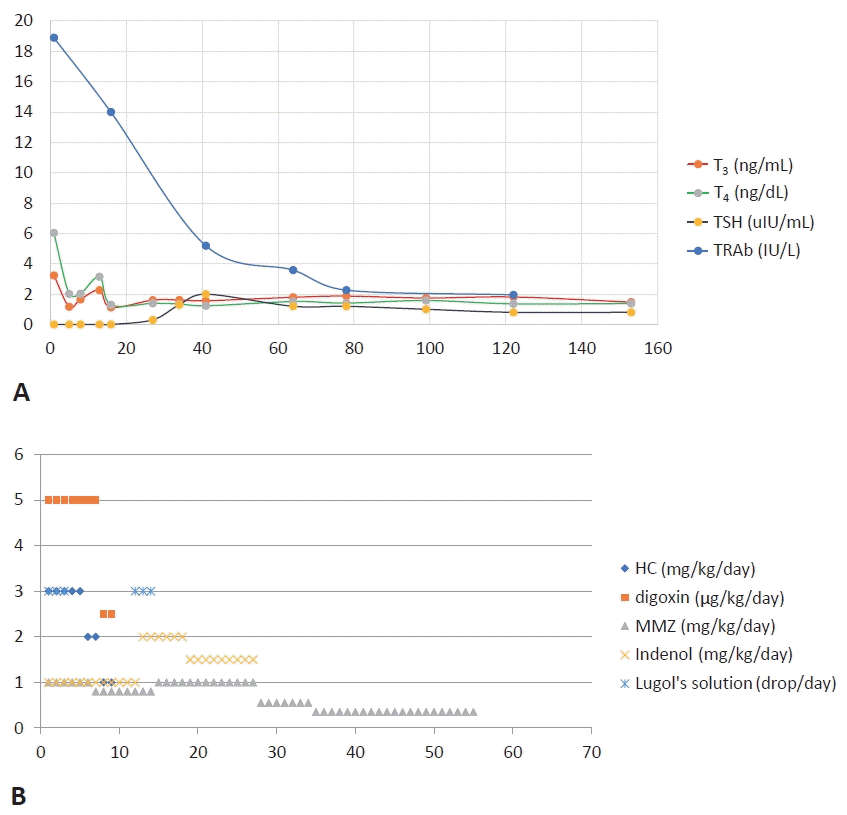
We started methimazole and digitalization at 1 and 5 μg/kg/day, respectively. Due to cardiac insufficiency, propranolol was started at 1 mg/kg/day. At 12 hours after admission, he appeared acute with decreased activity and pallor. He was unable to suck well with post-feeding apnea and cyanosis. He had shallow and rapid breathing (respiratory rate, 70/min) with hypoxia (saturation of oxygen, 90%) and tachycardia (pulse rate, 180/min). The patient was moved to an incubator in the neonatal intensive care unit. He was treated with high-flow nasal cannulation (3 L/min; fraction of inspired oxygen, 0.21). Additionally, we administered 1 drop of Lugol’s solution (Gemstein soln, Kolmar, Sejong, Korea), with 1:10 dilution in water three times a day and hydrocortisone 3 mg/kg/day. After administration of Lugol’s solution for 3 days, thyroid function showed improvement (FT4, 2.01 ng/dL; T3, 1.17 ng/mL; TSH, <0.1 μIU/mL). Therefore, Lugol’s solution was discontinued on day 4 of hospitalization. The thyroid hormone levels increased again (FT4, 3.15 ng/dL; T3, 2.26 ng/mL; TSH, <0.1 mU/mL) on day 11 of hospitalization. Therefore, Lugol’s solution was restarted. On day 14 of hospitalization, thyroid hormone levels were normalized (FT4, 1.3 ng/dL; T3, 1.13 ng/mL; TSH, <0.1 mU/mL) and Lugol’s solution was discontinued. Since then, there has been no increase in the thyroid hormone levels. Hydrocortisone was infused intravenously for 3 days from day 1 of hospitalization, it was reduced over 3 days, and subsequently discontinued (Figure 3).
As his general condition gradually stabilized, the dosage of methimazole was gradually reduced and discontinued at 75 days after birth and 54 days after treatment. At 6 months of age, he remains euthyroid (FT4, 1.35 ng/dL; T3, 1.47 ng/mL; TSH, 0.8 μUI/mL) with normal heart function (ejection fraction, 58.6%). His body weight was 9.9 kg (>97th percentile), height was 71.4 cm (>97th percentile), and head circumference was 45 cm (>97th percentile). The patient did not show any developmental delay.
The risk of fetal and neonatal hyperthyroidism increases if maternal TRAb level is elevated by more than two to three times the upper limit of normal during the second or third trimester of pregnancy [8]. A systemic review by van Dijk et al. [3] reported that the lowest TRAb level in mothers causing NGD was 4.4 U/L, which was 3.7 times the upper limit of normal.
TRAb may remain after past thyroidectomy or radioiodine treatment, cross the placenta, and affect the fetus and neonate [6]. NGD has also been reported to be associated with TSAb in mothers with Hashimoto’s thyroiditis [9].
Maternal thyrotropin-binding inhibitory immunoglobulin level >5 IU/L indicates a risk of fetal or neonatal hyperthyroidism. Among these mothers, TSAb measurement contributes to specific identification of those who require close follow-up. Maternal TSAb levels above 350% to 500% of the control are associated with a higher incidence of neonatal hyperthyroidism [10].
Fetal manifestations of hyperthyroidism include tachycardia, thyroid enlargement, intrauterine growth retardation, polyhydramnios or oligohydramnios, advanced bone age, craniosynostosis, microcephaly, and hydrops. Neonates may present with tachycardia, irritability, tremors, poor feeding, sweating, difficulty in sleeping, emaciated appearance, proptosis with stare, and goiter. Craniosynostosis and microcephaly may be observed in severely affected infants. Other rare signs that may be confused with infection/sepsis include thrombocytopenia, hepatosplenomegaly, and jaundice. Mortality rates of up to 20% have been reported and cardiac failure is the most common cause of death [1].
Careful examination and monitoring must be performed after birth. Neonates born to mothers who tested negative for TRAb during the second half of gestation or exhibited absence of TRAb in the cord blood are unlikely to develop GD and are considered low-risk patients. They can be followed clinically and thyroid function tests can be performed only if symptoms or signs of hyperthyroidism develop. Conversely, if the TRAb level of a mother with GD is elevated or unknown, the neonate is considered a high-risk patient [11].
The time of onset and severity of symptoms of hyperthyroidism are variable. Neonates born to mothers who had high TRAb levels (more than three times the upper normal value) and who were not treated with ATDs can exhibit overt hyperthyroidism at birth, while neonates born to mothers treated with ATDs or neonates who receive maternal TBAb may have normal thyroid function or even hypothyroidism at birth. With metabolization of the ATDs passed from the mother, hyperthyroidism may gradually develop within 2 to 5 days after birth [12].
Thyroid function tests performed on the cord blood or within 3 days of life in neonates often reflect maternal disease status rather than neonatal disease. Thus, these tests do not predict subsequent hyperthyroidism. Unless clinical signs warrant earlier investigations, thyroid function tests should be performed in the first week, especially between days 3 and 5 of life, even if normal or high TSH levels are observed in tests performed on cord blood [5,11]. Although maternal ATDs may delay the presentation of hyperthyroidism, most of the cases of hyperthyroidism are diagnosed within the first 2 weeks of life [11].
In Korea, Goh et al. [13] reported delayed onset of neonatal hyperthyroidism in an 11-day-old female patient born to a mother with GD. The patient showed hypothyroidism at 5 days of age (T4, 3.5 µg/dL; TSH, 501.74 μIU/mL) in a newborn screening test. She was referred for the evaluation of congenital hypothyroidism at 11 days of age. She looked healthy, but her thyroid function tests revealed hyperthyroidism (T4, 59.6 µg/dL [reference range, 6.0 to 15.9]; TSH, 0.43 μIU/mL; TRAb, 46.0% [reference range, <15]). Her mother had been taking propylthiouracil during pregnancy. It seems that the patient was hypothyroid at birth and became hyperthyroid with subsequent metabolization and excretion of maternal ATDs from her circulation. Oh et al. [14] reported a case of a 24-day old male patient with tachycardia, sweating, and irritability who was born to a mother with unrecognized GD. At 34 weeks of gestation, fetal tachycardia (160 to 180/min) was observed. He was delivered by cesarean section at 35 weeks and 3 days of gestation with a birth weight of 2,360 g. Even after birth, tachycardia persisted. Echocardiography and thyroid function tests were performed, but the results were normal. During followup at the outpatient clinic, he showed tachycardia, sweating, and irritability with increased FT4 (2.39 ng/dL) and decreased TSH (0.00 μIU/mL) at 24 days of age. The mother was diagnosed after the diagnosis of the patient. Our patient also exhibited TSH and T4 levels within the normal range in the screening test after birth. Nonetheless, serial follow-up was required considering the possibility of delayed symptoms, but it was not performed [11].
If neonatal hyperthyroidism is confirmed via clinical and biochemical evaluations, treatment should be initiated promptly. Methimazole is preferred (0.5 to 1 mg/kg/day) and propranolol (2 mg/kg/day) can be used to control neuromuscular and cardiovascular hyperactivity, which is the inhibition of T4 conversion to T3. Iodine (one drop of Lugol's solution every 8 hours orally) may be administered to neonates whose hyperthyroidism is not controlled with methimazole and propranolol. Glucocorticoids can also be administered to extremely ill patients. They inhibit thyroid hormone secretion and decrease peripheral conversion of T4 to T3 [5,11]. It is unclear whether asymptomatic newborns with biochemical hyperthyroidism should be treated.
Besancon et al. [4] reported that rapid FT4 elevation during the first postnatal week is predictive of hyperthyroidism and warrants ATD therapy. Banige et al. [15] reported that serum TSH level below 0.90 mU/L at 3 to 7 days of age predicted the development of hyperthyroidism in most of the cases. Leger [5] reported that hyperthyroidism is strongly suspected when TRAb are detectable and exhibit high levels (more than three times the upper limit of the normal range, generally >5 IU/L) in the cord blood and FT4 levels are high in the first 2 to 4 days after birth (above the upper limit of the normal range for age, ≥40 pmol/L or ≥3.1 ng/dL). In such cases, ATD treatment should be initiated in infants shortly after birth to prevent the development of clinical hyperthyroidism [5].
Since maternal TRAb are eliminated from the infant circulation, the disease may last for 1 to 3 months [5]. With adequate therapy, most of the neonates with hyperthyroidism improve rapidly. Nevertheless, there might be some long-term adverse effects on cognitive development even with prompt treatment [5,11]. Some individuals develop subsequent central hypothyroidism due to prenatal exposure of the hypothalamus and pituitary gland to high serum thyroid hormone concentrations [16,17].
In conclusion, all infants born to mothers with a history of GD should undergo careful examination and monitoring to prevent the development of clinical hyperthyroidism and serious complications associated with it. TRAb levels in neonates should be checked in the cord blood or as soon as possible after birth. If TRAb levels are detectable, thyroid function tests should be repeated during the first 2 weeks of life (at days 3, 5, 7, 10, and 15) and the patients should be followed up clinically until 2 to 3 months of life [1,11].
Since there might be no obvious symptoms of hyperthyroidism, maternal medical history and a high index of suspicion are key to making the diagnosis even when the thyroid function tests are normal for days after birth.
Notes
REFERENCES
1. Samuels SL, Namoc SM, Bauer AJ. Neonatal thyrotoxicosis. Clin Perinatol. 2018; 45:31–40.
2. Segni M. Neonatal hyperthyroidism. In : Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, editors. Endotext. South Dartmouth: MDText.com;2000. https://www.ncbi.nlm.nih.gov/books/NBK279019.
3. van Dijk MM, Smits IH, Fliers E, Bisschop PH. Maternal thyrotropin receptor antibody concentration and the risk of fetal and neonatal thyrotoxicosis: a systematic review. Thyroid. 2018; 28:257–64.
4. Besancon A, Beltrand J, Le Gac I, Luton D, Polak M. Management of neonates born to women with Graves’ disease: a cohort study. Eur J Endocrinol. 2014; 170:855–62.
5. Leger J. Management of fetal and neonatal Graves’ disease. Horm Res Paediatr. 2017; 87:1–6.
6. Volpe R, Ehrlich R, Steiner G, Row VV. Graves’ disease in pregnancy years after hypothyroidism with recurrent passive-transfer neonatal Graves’ disease in offspring: therapeutic considerations. Am J Med. 1984; 77:572–8.
7. Perry RJ, Hollman AS, Wood AM, Donaldson MD. Ultrasound of the thyroid gland in the newborn: normative data. Arch Dis Child Fetal Neonatal Ed. 2002; 87:F209–11.
8. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017; 27:315–89.
9. Kiefer FW, Klebermass-Schrehof K, Steiner M, Worda C, Kasprian G, Diana T, et al. Fetal/neonatal thyrotoxicosis in a newborn from a hypothyroid woman with Hashimoto thyroiditis. J Clin Endocrinol Metab. 2017; 102:6–9.
10. Abeillon-du Payrat J, Chikh K, Bossard N, Bretones P, Gaucherand P, Claris O, et al. Predictive value of maternal secondgeneration thyroid-binding inhibitory immunoglobulin assay for neonatal autoimmune hyperthyroidism. Eur J Endocrinol. 2014; 171:451–60.
11. van der Kaay DC, Wasserman JD, Palmert MR. Management of neonates born to mothers with Graves’ disease. Pediatrics. 2016; 137:e20151878.
12. Zakarija M, McKenzie JM, Hoffman WH. Prediction and therapy of intrauterine and late-onset neonatal hyperthyroidism. J Clin Endocrinol Metab. 1986; 62:368–71.
13. Goh J, Cho HS, Oh PS, Cha JK, Kim JW, Park CY, et al. A case of neonatal Graveses disease. J Korean Soc Pediatr Endocrinol. 1999; 4:104–8.
14. Oh SM, Kim HS, Sung TJ, Yang S. A case of neonatal thyrotoxicosis detected by neonatal tachycardia. Korean J Perinatol. 2008; 19:80–3.
15. Banige M, Polak M, Luton D; Research Group for Perinatal Dysthyroidism (RGPD) Study Group. Prediction of neonatal hyperthyroidism. J Pediatr. 2018; 197:249–54.
16. Kempers MJ, van Trotsenburg AS, van Rijn RR, Smets AM, Smit BJ, de Vijlder JJ, et al. Loss of integrity of thyroid morphology and function in children born to mothers with inadequately treated Graves’ disease. J Clin Endocrinol Metab. 2007; 92:2984–91.
17. Kempers MJ, van Tijn DA, van Trotsenburg AS, de Vijlder JJ, Wiedijk BM, Vulsma T. Central congenital hypothyroidism due to gestational hyperthyroidism: detection where prevention failed. J Clin Endocrinol Metab. 2003; 88:5851–7.
Figure 1.
Infantogram shows cardiomegaly. It can rule out lung problems from the possible causes of tachypnea and tachycardia.
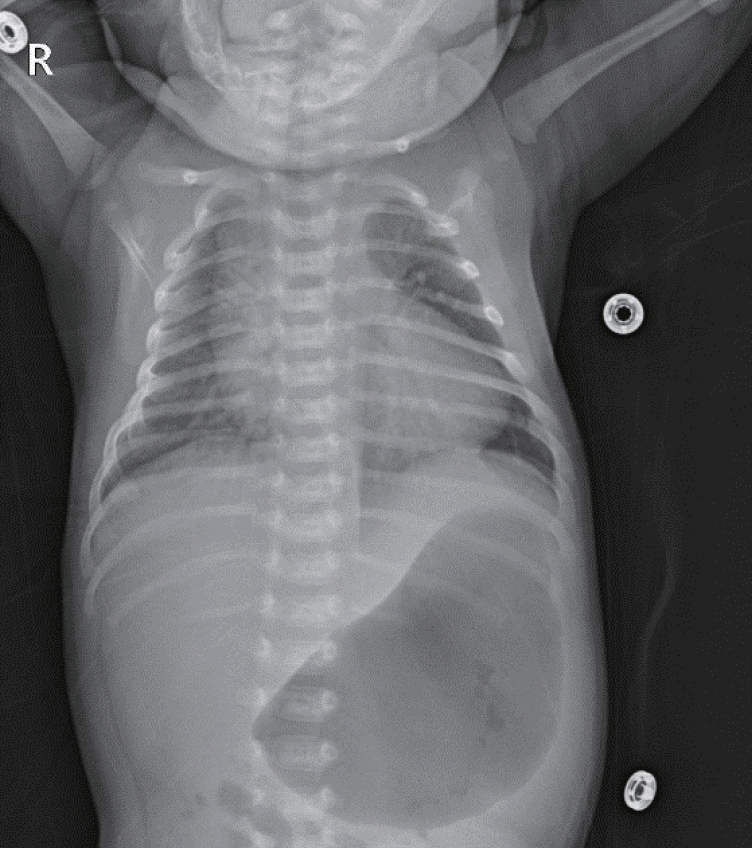
Figure 2.
Both the thyroid lobes were enlarged and coarse on ultrasound. (A) The breadth and depth of the right lobe were 17.11 and 11.58 mm, respectively. (B) The breadth and depth of the left lobe were 15.86 and 11.20 mm, respectively. (C) The length estimated by Tc-99m pertechnetate scan was 29.3 mm and uptake was increased. This corresponds to 1.86 times the mean thyroid gland volume of a normal newborn (thyroid volume=length×breadth×depth×π/6) [7].
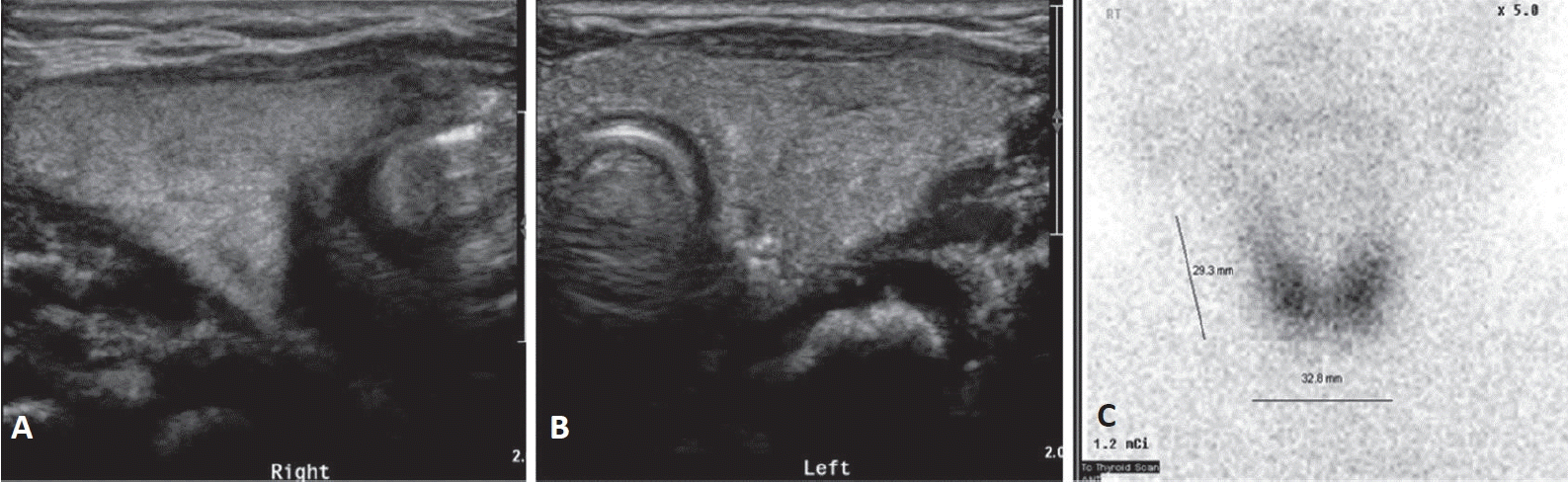
Figure 3.
Trend of (A) thyroid function test and (B) treatment. The thyroid hormone was normalized on day 14 of hospitalization. However, the level of thyroid-stimulating hormone (TSH) receptor antibodies (TRAb) was decreased to 0.8 IU/L at 8 months of age. Day 1 of treatment was day 21 after birth. Abbreviations: T3, triiodothyronine; FT4, free thyroxine; HC, hydrocortisone; MMZ, methimazole.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download