1. Blencowe H, Lee AC, Cousens S, Bahalim A, Narwal R, Zhong N, et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res. 2013; 74 Suppl 1:17–34.
2. Sutton PS, Darmstadt GL. Preterm birth and neurodevelopment: a review of outcomes and recommendations for early identification and cost-effective interventions. J Trop Pediatr. 2013; 59:258–65.
3. Banihani R, Seesahai J, Asztalos E, Terrien Church P. Neuroimaging at term equivalent age: is there value for the preterm infant? A narrative summary. Children (Basel). 2021; 8:227.
4. Antonov NK, Ruzal-Shapiro CB, Morel KD, Millar WS, Kashyap S, Lauren CT, et al. Feed and wrap MRI technique in infants. Clin Pediatr (Phila). 2017; 56:1095–103.
5. Eker HE, Cok OY, Cetinkaya B, Aribogan A. Oral 30% glucose provides sufficient sedation in newborns during MRI. J Anesth. 2017; 31:206–11.
6. Parad RB. Non-sedation of the neonate for radiologic procedures. Pediatr Radiol. 2018; 48:524–30.
7. Boutros A, Pavlicek W. Anesthesia for magnetic resonance imaging. Anesth Analg. 1987; 66:367.
8. Hupp JR. Pediatric anesthesia: concerns about neurotoxicity. J Oral Maxillofac Surg. 2015; 73:1021–2.
9. Durrmeyer X, Vutskits L, Anand KJ, Rimensberger PC. Use of analgesic and sedative drugs in the NICU: integrating clinical trials and laboratory data. Pediatr Res. 2010; 67:117–27.
10. Sun L. Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth. 2010; 105 Suppl 1:i61–8.
11. Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanic K, et al. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012; 87:120–9.
12. DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011; 113:1143–51.
13. Cote CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics. 2000; 106:633–44.
14. Havidich JE, Beach M, Dierdorf SF, Onega T, Suresh G, Cravero JP. Preterm versus term children: analysis of sedation/anesthesia adverse events and longitudinal risk. Pediatrics. 2016; 137:e20150463.
15. Haney B, Reavey D, Atchison L, Poull J, Dryer L, Anderson B, et al. Magnetic resonance imaging studies without sedation in the neonatal intensive care unit: safe and efficient. J Perinat Neonatal Nurs. 2010; 24:256–66.
16. Ureta-Velasco N, Martinez-de Aragon A, Moral-Pumarega MT, Nunez-Enamorado N, Bergon-Sendin E, Pallas-Alonso CR. Magnetic resonance imaging without sedation in neonates. An Pediatr (Barc). 2015; 82:354–9.
17. Finnemore A, Toulmin H, Merchant N, Arichi T, Tusor N, Cox D, et al. Chloral hydrate sedation for magnetic resonance imaging in newborn infants. Paediatr Anaesth. 2014; 24:190–5.
18. Executive summary: neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists' Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014; 123:896–901.
19. Cote CJ, Wilson S; American Academy of Pediatrics; American Academy of Pediatric Dentistry. Guidelines for monitoring and management of pediatric patients before, during, and after sedation for diagnostic and therapeutic procedures: update 2016. Pediatrics. 2016; 138:e20161212.
20. Davidson AJ, Becke K, de Graaff J, Giribaldi G, Habre W, Hansen T, et al. Anesthesia and the developing brain: a way forward for clinical research. Paediatr Anaesth. 2015; 25:447–52.
21. Gleich SJ, Flick R, Hu D, Zaccariello MJ, Colligan RC, Katusic SK, et al. Neurodevelopment of children exposed to anesthesia: design of the Mayo Anesthesia Safety in Kids (MASK) study. Contemp Clin Trials. 2015; 41:45–54.
22. Fogel MA, Pawlowski TW, Harris MA, Whitehead KK, Keller MS, Wilson J, et al. Comparison and usefulness of cardiac magnetic resonance versus computed tomography in infants six months of age or younger with aortic arch anomalies without deep sedation or anesthesia. Am J Cardiol. 2011; 108:120–5.
23. Golan A, Marco R, Raz H, Shany E. Imaging in the newborn: infant immobilizer obviates the need for anesthesia. Isr Med Assoc J. 2011; 13:663–5.
24. Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 2008; 38:260–4.
25. Shariat M, Mertens L, Seed M, Grosse-Wortmann L, Golding F, Mercer-Rosa L, et al. Utility of feed-and-sleep cardiovascular magnetic resonance in young infants with complex cardiovascular disease. Pediatr Cardiol. 2015; 36:809–12.
26. Windram J, Grosse-Wortmann L, Shariat M, Greer ML, Crawford MW, Yoo SJ. Cardiovascular MRI without sedation or general anesthesia using a feed-and-sleep technique in neonates and infants. Pediatr Radiol. 2012; 42:183–7.
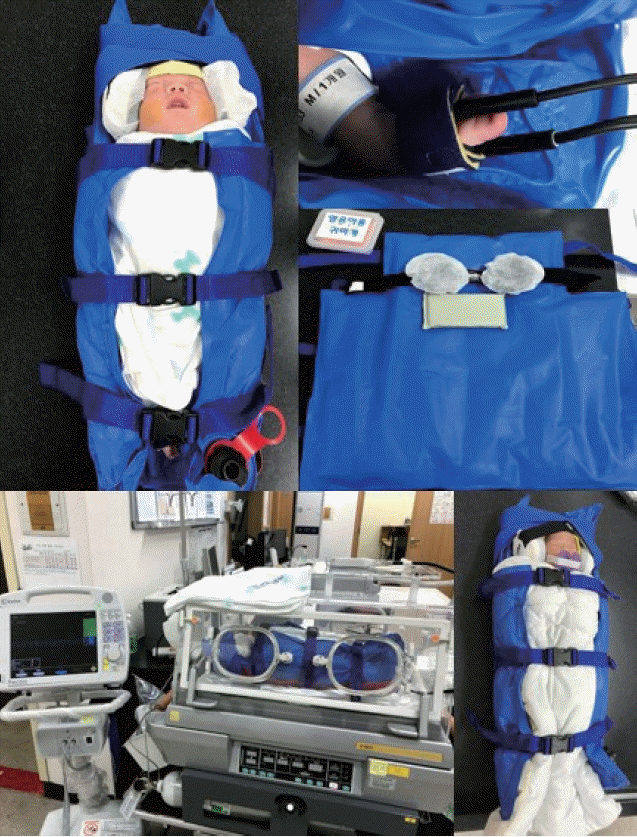
27. Yamamura K, Takatsu Y, Miyati T, Inatomi T. Brain magnetic resonance imaging using a customized vacuum shape-keeping immobilizer without sedation in preterm infants. Magn Reson Imaging. 2018; 54:171–5.
28. Heller BJ, Yudkowitz FS, Lipson S. Can we reduce anesthesia exposure? Neonatal brain MRI: swaddling vs. sedation, a national survey. J Clin Anesth. 2017; 38:119–22.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download