2. Hoeffler CD, Krenek ME, Brand MC. Wolff-Parkinson-White syndrome in a term infant presenting with cardiopulmonary arrest. Adv Neonatal Care. 2016; 16:44–51.
3. Tsao S, Deal BJ. Management of symptomatic Wolff-Parkinson- White syndrome in childhood. Prog Pediatr Cardiol. 2013; 35:7–15.
4. Dharnidharka VR, Lieh-Lai M, Sarnaik A, Clapp S. A child with cardiogenic shock and supraventricular tachycardia presenting in normal sinus rhythm. Pediatr Emerg Care. 1996; 12:420–1.
5. Gikonyo BM, Dunnigan A, Benson DW Jr. Cardiovascular collapse in infants: association with paroxysmal atrial tachycardia. Pediatrics. 1985; 76:922–6.
6. Viveiros E, Aveiro AC, Costa E, Nunes JL. Cardiogenic shock in a neonate. BMJ Case Rep. 2013; 2013:bcr2012008440.
7. Al-Khatib SM, Pritchett EL. Clinical features of Wolff-Parkinson- White syndrome. Am Heart J. 1999; 138(3 Pt 1):403–13.
8. Munger TM, Packer DL, Hammill SC, Feldman BJ, Bailey KR, Ballard DJ, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953-1989. Circulation. 1993; 87:866–73.
9. Richardson C, Silver ES. Management of supraventricular tachycardia in infants. Paediatr Drugs. 2017; 19:539–51.
10. MacRae CA, Ghaisas N, Kass S, Donnelly S, Basson CT, Watkins HC, et al. Familial hypertrophic cardiomyopathy with Wolff-Parkinson-White syndrome maps to a locus on chromosome 7q3. J Clin Invest. 1995; 96:1216–20.
11. van der Steld LP, Campuzano O, Perez-Serra A, Moura de Barros Zamorano M, Sousa Matos S, Brugada R. Wolff-Parkinson-White syndrome with ventricular hypertrophy in a Brazilian family. Am J Case Rep. 2017; 18:766–76.
12. Gilljam T, Jaeggi E, Gow RM. Neonatal supraventricular tachycardia: outcomes over a 27-year period at a single institution. Acta Paediatr. 2008; 97:1035–9.
13. Riggs TW, Byrd JA, Weinhouse E. Recurrence risk of supraventricular tachycardia in pediatric patients. Cardiology. 1999; 91:25–30.
14. National Center for Biotechnology Information, U.S. National Library of Medicine. MYL2 myosin light chain 2 [homo sapiens (human)] [Internet]. Bethesda: NCBI Gene;2020. [cited 2021 May 12]. Available from:
http://www.ncbi.nlm.nih.gov/gene/4633.
15. Andersen PS, Havndrup O, Hougs L, Sorensen KM, Jensen M, Larsen LA, et al. Diagnostic yield, interpretation, and clinical utility of mutation screening of sarcomere encoding genes in Danish hypertrophic cardiomyopathy patients and relatives. Hum Mutat. 2009; 30:363–70.
16. Claes GR, van Tienen FH, Lindsey P, Krapels IP, Helderman-van den Enden AT, Hoos MB, et al. Hypertrophic remodelling in cardiac regulatory myosin light chain (MYL2) founder mutation carriers. Eur Heart J. 2016; 37:1815–22.
17. Ho CY, Day SM, Colan SD, Russe ll MW, Towbin JA, Sherrid MV, et al. The burden of early phenotypes and the influence of wall thickness in hypertrophic cardiomyopathy mutation carriers: findings from the HCMNet study. JAMA Cardiol. 2017; 2:419–28.
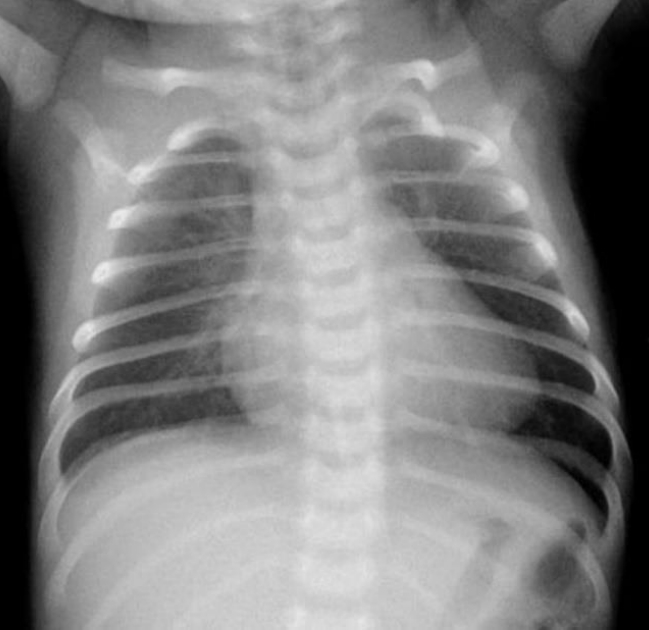
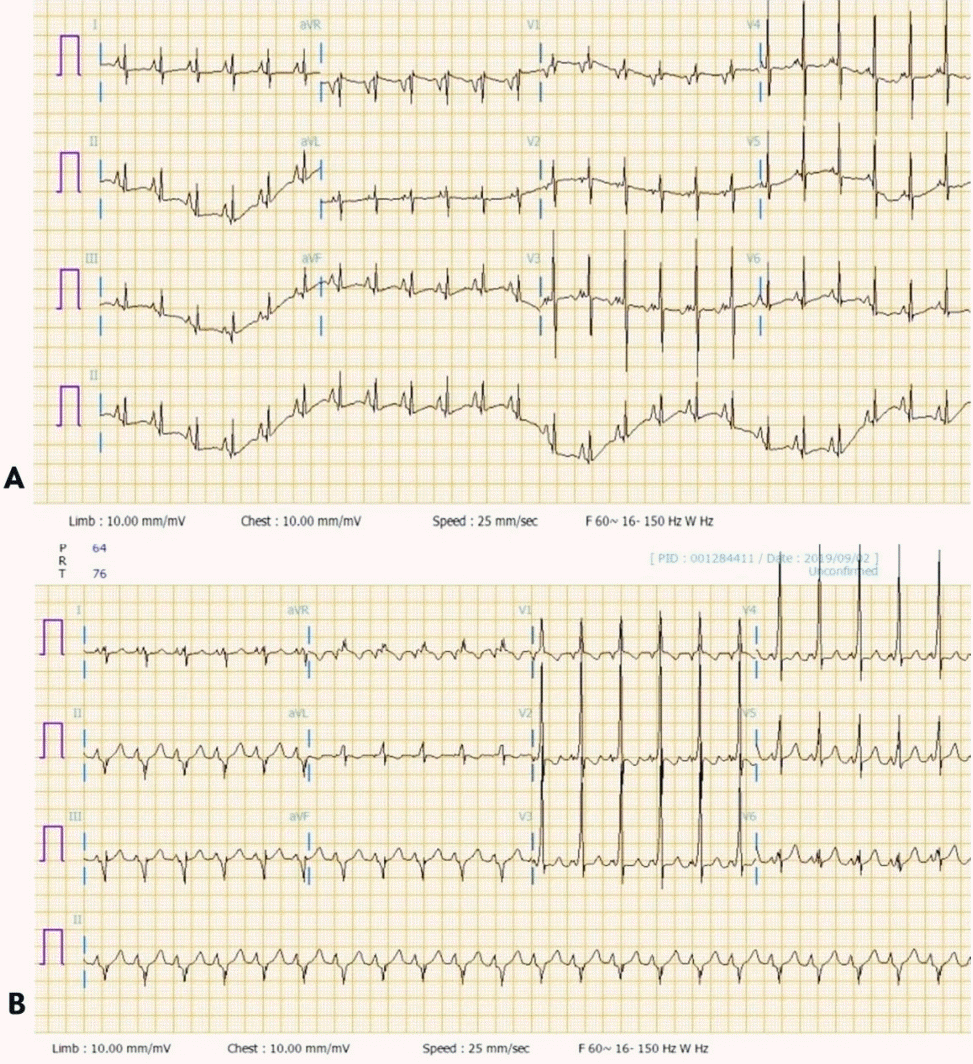




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download