Abstract
Iatrogenic esophageal perforation is a rare condition in children, but occasionally occurs in premature infants due to repeated intubation or nasogastric tube insertion. Esophageal perforation is common in pneumothorax and interstitial emphysema, but rarely occurs in the absence of pneumothorax. Although complications, such as mediastinitis, after esophageal perforation are rare, they can be fatal. Therefore, rapid and accurate diagnosis and appropriate treatment are important. The authors report a case of huge retrocardiac pneumomediastinum and subcutaneous emphysema suspected to be caused by esophageal perforation after repeated intubation.
Iatrogenic esophageal perforation is a rare condition in neonates, but can often be encountered in preterm or low birth weight infants due to repeated intubation or nasogastric tube insertion after birth [1]. In neonates, the injury site is usually at the pharyngoesophageal junction, proximal to the cricopharyngeal muscle [2,3].
Premature neonates are unique in that they manifest different signs and symptoms, such as hypersalivation, choking, coughing, or cyanosis, after repeated or difficult attempts at endotracheal or enterogastric intubation [2,4].
Esophageal perforation can cause the spread of bacterial or digestive enzymes to the mediastinum or sub-diaphragmatic space, which can occasionally cause mediastinitis, empyema, and/or sepsis [5]. Therefore, although esophageal perforation is very rare, rapid and accurate diagnosis and appropriate treatment are important because such a condition can be fatal. There have been relatively few published experiences describing the treatment of esophageal perforation, especially in cases that led to pneumomediastinum in neonates. We describe a case of huge retrocardiac pneumomediastinum and subcutaneous emphysema suspected to be caused by esophageal perforation after repeated intubation.
A baby girl was born at 31 weeks’ gestation by caesarean section due to bag bulging with one arm out. Her birth weight was 1,362 g (25th percentile), and her birth length was 36 cm (<10th percentile). Within a few minutes after birth, the baby exhibited respiratory distress with cyanosis, and an increasing oxygen requirement (oxygen saturation 62% on room air). In the operating room, intubation was successfully performed without placing an oro- or nasogastric tube. Apgar score was 4 at 1 minute and 6 at 5 minutes. Fifteen minutes after being admitted to the neonatal intensive care unit, the endotracheal tube was spontaneously removed by accident. After three failed attempts, reintubation was successful on the fourth, and a nasogastric tube was also inserted. Chest X-ray performed after endotracheal tube placement revealed ground-glass opacities in both lung fields, and a small quantity of air in the mediastinum, with air tracking to the right neck. Respiratory distress syndrome and pneumomediastinum with subcutaneous emphysema were diagnosed. Surfactant was administrated and oxygen was supplied at a high rate for 1 hour (Figure 1). On day 3 of admission, a follow-up X-ray revealed pneumomediastinum extending to the back of middle thoracic esophagus. On day 7, pneumomediastinum had extended further into the posterior portion of the lower esophagus, with cervical subcutaneous emphysema evident on X-ray (Figure 2). Chest computed tomography (CT) was performed and revealed that the pneumomediastinum had extended to all areas of the mediastinum. Subcutaneous emphysema around the neck remained evident (Figure 3). On chest CT, the orogastric tube was near the pneumomediastinum (Figure 3A). There was retrocardiac pneumoperitoneum with cervical subcutaneous emphysema but no pneumothorax or interstitial emphysema; accordingly, the baby was diagnosed with isolated pneumomediastinum.
She was treated conservatively without primary surgery or thoracic drainage because her vital signs were relatively stable. She was kept on mechanical ventilation for 7 to 10 days using high-frequency oscillation and pressure support ventilation with low pressures, and broad-spectrum antibiotics were administered for 2 weeks. Attempts were made to reduce the frequency of orogastric suction as much as possible. Nutritional support was started in the form of total parenteral nutrition, with trophic feeding through the orogastric tube.
Respiratory distress syndrome improved from day 4 of admission and subcutaneous emphysema disappeared around day 7. No false track was observed on chest CT performed on day 7 of admission. Laryngo fiberscopy was additionally performed to confirm that there was no injury to the larynx. On day 9 of admission, the endotracheal tube was removed. The huge pneumomediastinum gradually decreased and improved at around 10 days after admission. The baby was gradually weaned off respiratory support. Oral feeding was introduced and gradually increased to full feeds. One full feeding was tolerated on day 74 of admission, and she was discharged.
Esophageal perforation is a rare condition in newborns; however, it is more common in premature infants requiring resuscitation using tracheal intubation [6,7]. Neonates most at risk include small for gestational age or premature infants [8]. The overall estimated incidence of iatrogenic esophageal perforation is 0.8% in preterm infants. However, this incidence has been reported to be as high as 4% in newborns weighing <750 g [9]. The most common causes of iatrogenic esophageal perforation include nasogastric tube insertion, endotracheal intubation, and nasotracheal suction2 [10].
Signs and symptoms of esophageal perforation may vary initially and depend on the site of esophageal injury [11,12]. Cervical perforation presents with neck pain, cervical dysphagia, dysphonia, or bloody regurgitation and subcutaneous emphysema [13]. Subcutaneous emphysema in the soft tissue of the neck is the most common finding in cervical perforation and indicates leaked air tracking within the subcutaneous plane [14]. Full-term and premature infants present unique and different signs and symptoms [4]. A diagnosis of esophageal perforation should be considered in any infant presenting with hypersalivation, choking, coughing, or cyanosis after repeated or difficult attempts at endotracheal intubation [3].
In neonates, the site of injury is often at the pharyngoesophageal junction, proximal to the cricopharyngeal muscle [2]. Because the cricopharyngeal junction is the narrowest part of the esophagus, inexperienced manipulation of the laryngoscope blade or repeated suctioning can cause reflex of muscular constriction, which can lead to closure of the esophageal lumen. Extension of the neck compressing on the posterior esophageal wall against the cervical vertebrae can be another cause of this injury. Therefore, inattentive intubation at this time may lead to perforation of the esophagus [6,7].
Early diagnosis of esophageal perforation is critical and ensures the best possible outcome. Initial diagnosis should be made using plain X-ray. Treatment depends on the type and extent of injury, as well as the general condition of the infant [7]. Despite the improvement in critical care management, the most important factor in reducing mortality appears to be the time to treatment [15]. Esophageal perforation in children can be adequately managed with conservative treatment [16].
As a conservative treatment for esophageal perforation, the orogastric tube should be removed. Furthermore, electrolyte balance must be monitored closely, and appropriate antibiotics should be administered to prevent dehydration and shock
In our case, retrocardiac pneumomediastinum was observed behind the cricopharyngeal junction and cervical esophagus on day 1 of admission. Pneumomediastinum had extended and surrounded the thoracic esophagus by day 3 of admission. However, pneumothorax was not evident on plain X-ray and CT. Pneumomediastinum caused by pulmonary barotrauma occurs when the increase in alveolar pressure causes alveoli to rupture, subsequently releasing air, which in turn migrates through the peribronchial and perivascular sheaths to the mediastinum [17]. In infants with retrocardiac pneumomediastinum due to pressure impairment, extra-alveolar air, such as pneumothorax, interstitial emphysema, and pneumoperitoneum, is associated with a high incidence on chest radiography [18].
Another possible explanation for pneumomediastinum is the abnormal increase in pressure in the mediastinum. Pneumomediastinum is subjected to low and negative pressure similar to the pleural cavity, and causes the air to dissect mediastinal structures that support the mediastinal organs. A dramatic decrease in intravascular pressure also can create a relative pressure gradient in the perivascular spaces. The air may then dissect to the neck, upper abdomen or the skin via loose alveolar fat tissue (subcutaneous emphysema) [19].
Therefore, chest radiographs with pneumomediastinum and subcutaneous emphysema but without pneumothorax are findings indicative of esophageal perforation [7]. The absence of pneumothorax and interstitial emphysema, despite the presence of large amounts of retrocardiac pneumomediastinum, suggests that the cause is not necessarily due to pulmonary barotrauma [20]. Although CT could not confirm the perforation site, we were able to suspect esophageal perforation at the pharyngoesophageal junction from the fact that intubation was successful after several failed attempts, and from the retrocardiac pneumomediastinum behind the cricopharyngeal area and cervical esophagus subcutaneous emphysema observed on X-ray on day 1 of admission. In our case, non-operative management with parenteral nutrition, electrolyte replacement, fluid supplementation, respiratory support, and antibiotics was successful. In other cases of neonatal esophageal perforation reported to date, most were caused by misplacement of a nasogastric tube, which was found on plain X-ray. In contrast, our case was caused by repeated attempts at intubation, and the huge pneumomediastinum and subcutaneous emphysema without pneumothorax were identified on X-ray.
In conclusion, esophageal perforation is a rare complication in neonates, but can occur in inexperienced hands, and is associated with significant morbidity or mortality. As in our case, although not confirmed on radiographic imaging, if pneumomediastinum and subcutaneous emphysema develop without pneumothorax after repeated intubation, the possibility of esophageal perforation should be considered for rapid and accurate diagnosis and appropriate treatment.
Notes
REFERENCES
1. Emil SG. Neonatal esophageal perforation. J Pediatr Surg. 2004; 39:1296–8.
2. Sapin E, Gumpert L, Bonnard A, Carricaburu E, Sava E, Contencin P, et al. Iatrogenic pharyngoesophageal perforation in premature infants. Eur J Pediatr Surg. 2000; 10:83–7.
3. Hesketh AJ, Behr CA, Soffer SZ, Hong AR, Glick RD. Neonatal esophageal perforation: nonoperative management. J Surg Res. 2015; 198:1–6.
4. Gander JW, Berdon WE, Cowles RA. Iatrogenic esophageal perforation in children. Pediatr Surg Int. 2009; 25:395–401.
5. Stapp J, Stewart DL, Eberly S. Atypical latrogenic perforation of the distal esophagus in an ELBW neonate. Clin Pediatr (Phila). 2001; 40:637–8.
6. Garey CL, Laituri CA, Kaye AJ, Ostlie DJ, Snyder CL, Holcomb GW 3rd, et al. Esophageal perforation in children: a review of one institution's experience. J Surg Res. 2010; 164:13–7.
7. Rentea RM, St Peter SD. Neonatal and pediatric esophageal perforation. Semin Pediatr Surg. 2017; 26:87–94.
8. Al-Khawahur HA, Al-Salem AH. Iatrogenic perforation of the esophagus. Saudi Med J. 2002; 23:732–4.
9. Filippi L, Pezzati M, Poggi C. Use of polyvinyl feeding tubes and iatrogenic pharyngo-oesophageal perforation in very-low-birthweight infants. Acta Paediatr. 2005; 94:1825–8.
10. Soong WJ. Endoscopic diagnosis and management of iatrogenic cervical esophageal perforation in extremely premature infants. J Chin Med Assoc. 2007; 70:171–5.
11. Seefelder C, Elango S, Rosbe KW, Jennings RW. Oesophageal perforation presenting as oesophageal atresia in a premature neonate following difficult intubation. Paediatr Anaesth. 2001; 11:112–8.
12. Pumberger W, Bader T, Golej J, Pokieser P, Semsroth M. Traumatic pharyngo-oesophageal perforation in the newborn: a condition mimicking oesophageal atresia. Paediatr Anaesth. 2000; 10:201–5.
13. Panieri E, Millar AJ, Rode H, Brown RA, Cywes S. Iatrogenic esophageal perforation in children: patterns of injury, presentation, management, and outcome. J Pediatr Surg. 1996; 31:890–5.
14. Warden HD, Mucha SJ. Esophageal perforation due to trauma in the newborn. A case report. Arch Surg. 1961; 83:813–5.
15. Jones WG 2nd, Ginsberg RJ. Esophageal perforation: a continuing challenge. Ann Thorac Surg. 1992; 53:534–43.
16. Blair GK, Filler RM, Theodorescu D. Neonatal pharyngoesophageal perforation mimicking esophageal atresia: clues to diagnosis. J Pediatr Surg. 1987; 22:770–4.
17. Macklin MT, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: an interpretation of the clinical literature in the light of laboratory experiment. Medicine. 1944; 23:281–358.
18. Rosenfeld DL, Cordell CE, Jadeja N. Retrocardiac pneumomediastinum: radiographic finding and clinical implications. Pediatrics. 1990; 85:92–7.
19. Agut A, Talavera J, Buendia A, Anson A, Santarelli G, Gomez S. Imaging diagnosis-spontaneous pneumomediastinum secondary to primary pulmonary pathology in a dalmatian dog. Vet Radiol Ultrasound. 2015; 56:E54–7.
20. Amodio JB, Berdon WE, Abramson SJ, Oh KS, Oudjhane K, Wung JT. Retrocardiac pneumomediastinum in association with tracheal and esophageal perforations. Pediatr Radiol. 1986; 16:380–3.
Figure 1.
Chest radiographs of a premature infant. Chest radiographs captured on the day of birth (A, B) reveal ground-glass opacities in both lung fields and a small quantity of air in the mediastinum.
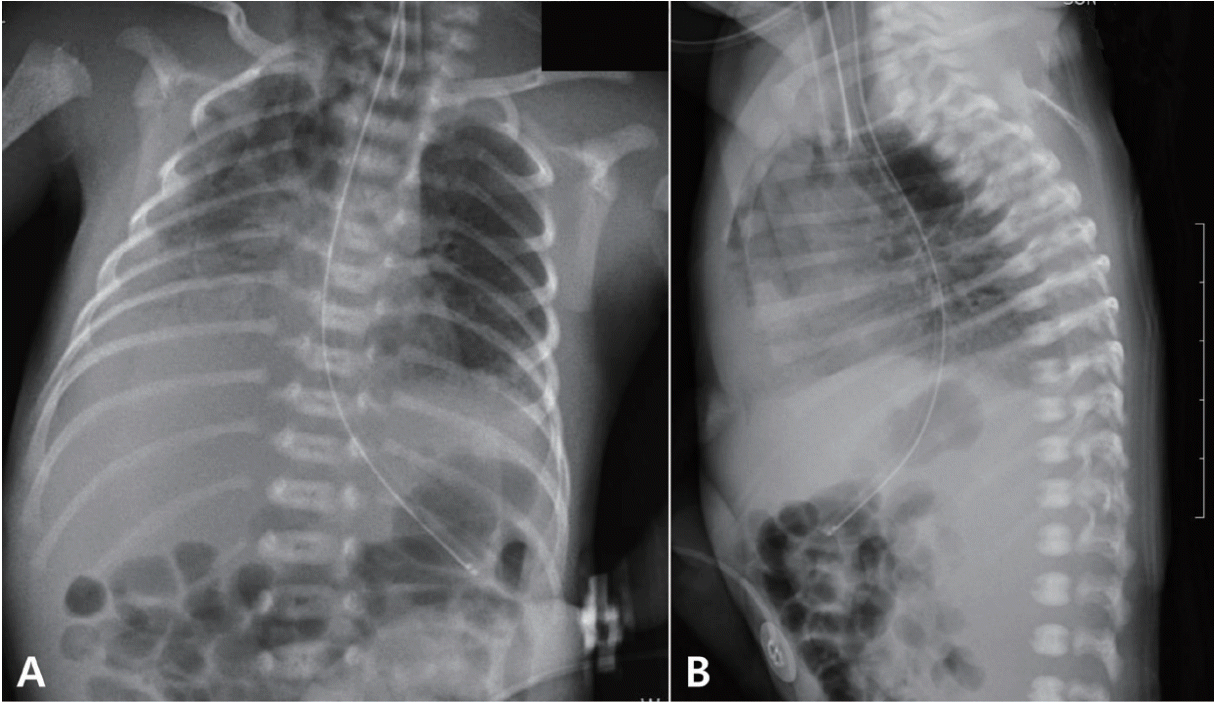
Figure 2.
Chest radiographs of a premature infant. Chest radiographs captured 7 days after birth (A, B) reveal extended radiolucency at both paravertebral areas (arrow), suggesting pneumomediastinum extending to the middle thoracic esophagus.
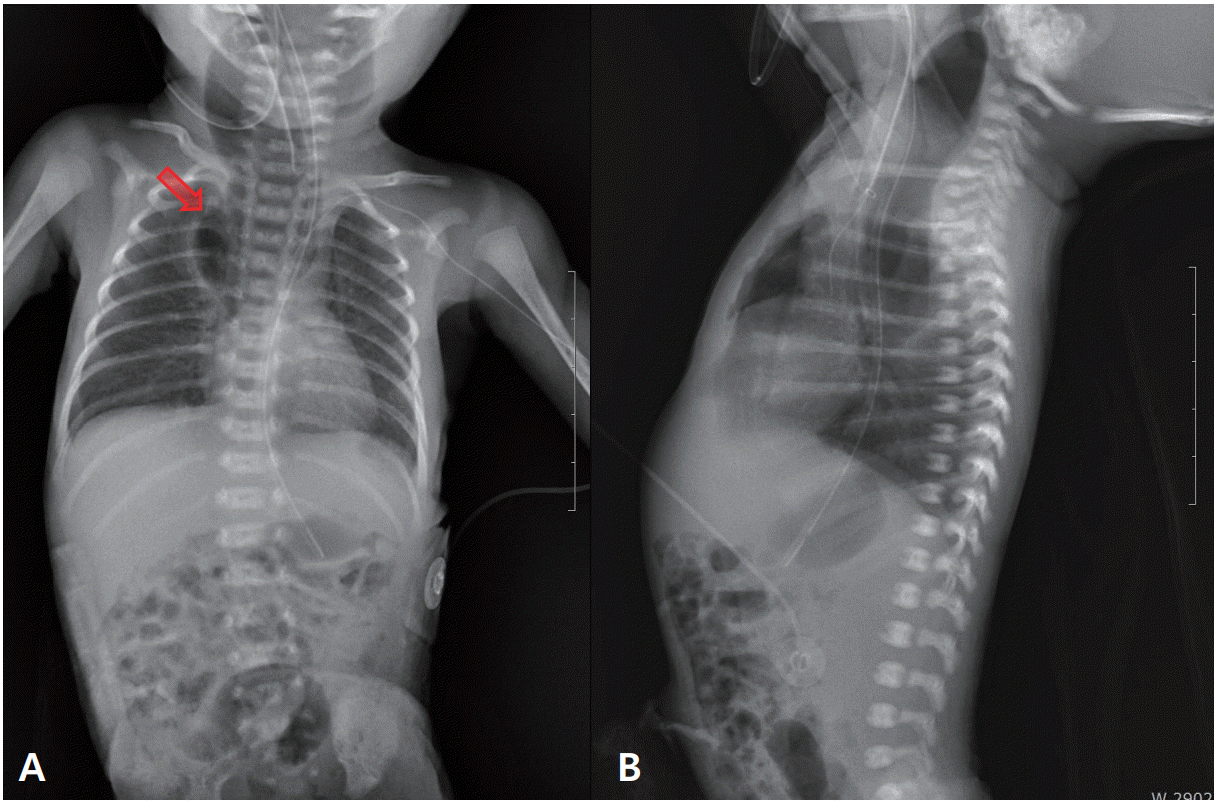
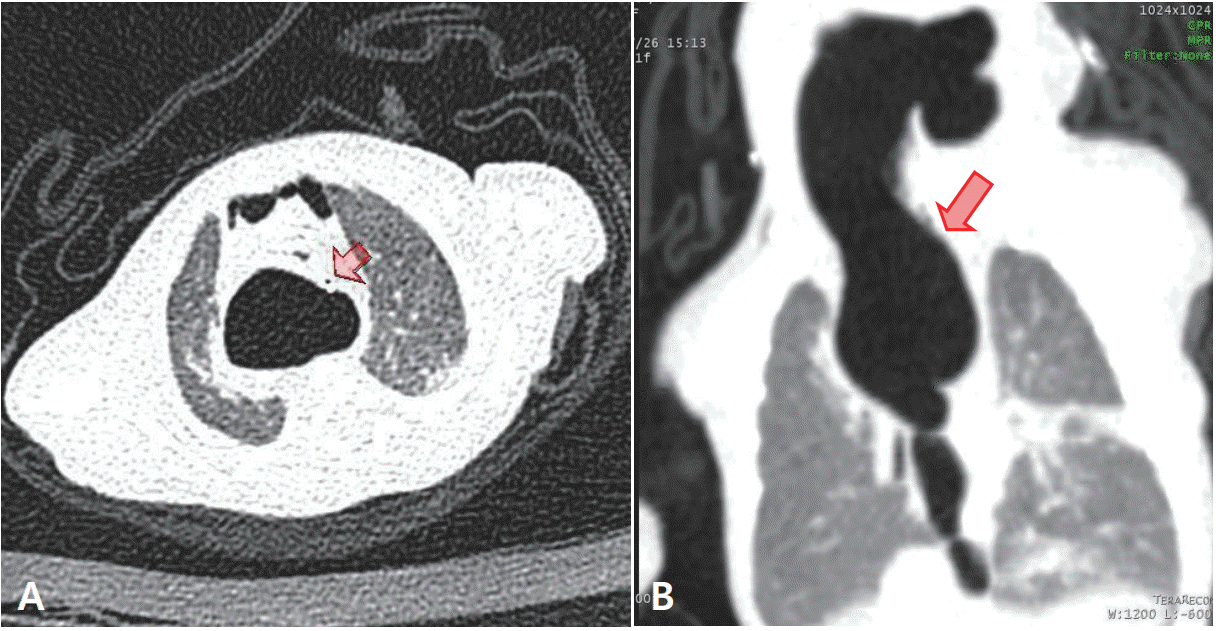
Figure 3.
Chest computed tomography (CT) scans of a premature infant. Axial (A) and coronal (B) CT scans performed 7 days after birth reveal huge retrocardiac pneumomediastinum surrounding the esophagus and extending to the diaphragm (arrow). The orogastric tube is evident near the pneumomediastinum (arrow).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download