INTRODUCTION
MATERIALS AND METHODS
1. Study design
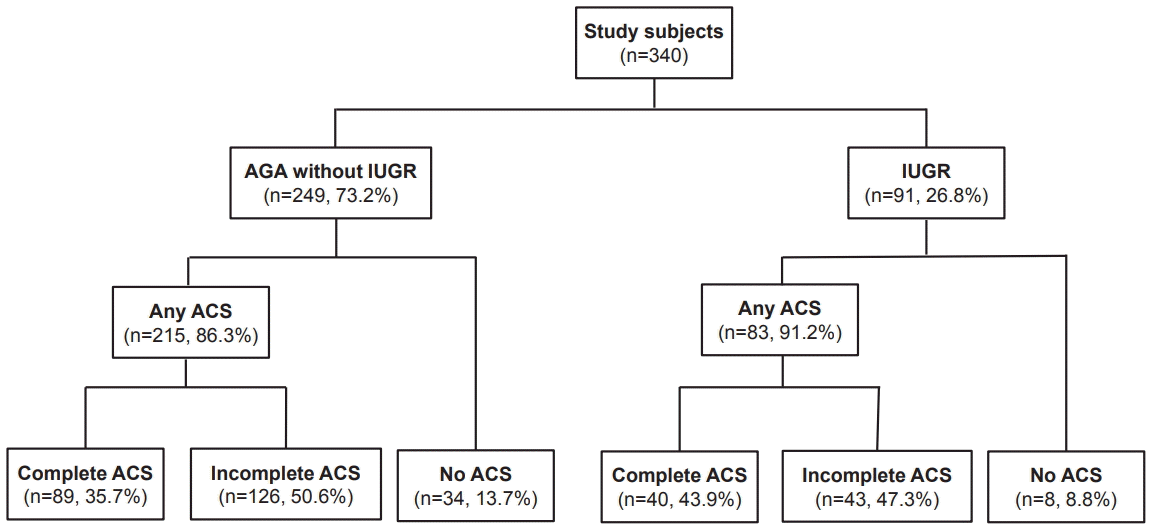 | Figure 1.Flow chart of the study population. A total of 340 singleton infants born at 23+0 to 33+6 weeks age of gestation between January 2007 and December 2014, satisfying the inclusion criteria of eligibility for this study. There was no significant difference in the proportion of each group divided according to antenatal corticosteroid (ACS) use (P=0.271). Abbreviations: AGA, appropriate for gestational age; IUGR, intrauterine growth restriction. |
2. Definitions
3. Statistical analysis
RESULTS
1. Demographic and baseline characteristics
Table 1.
| Characteristic |
AGA without IUGR |
IUGR |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=249) | Complete ACS (n=89, 35.7%) | Incomplete ACS (n=126, 50.6%) | No ACS (n=34, 13.7%) | P-value | Total (n=91) | Complete ACS (n=40,43.9%) | Incomplete (n=43,47.3%) | No ACS (n=8, 8.8%) | P-value | |
| GA (wk) | 30+4 (27+5–32+6) | 31+1 (27+3–32+5) | 30+2 (28+1–32+6) | 31+0 (25+5–33+1) | 0.958 | 30+1 (28+2–31+4) | 30+2 (29+1–32+2) | 29+2 (27+5–31+0) | 30+2 (27+4–32+5) | 0.092 |
| Birthweight (g) | 1,480 (990–1,890) | 1,510 (945–1,860) | 1,440 (1,098–1,843) | 1,560 (729–2,055) | 0.954 | 840 (640–1,040) | 890 (720–1,135) | 750 (590–940) | 920 (708–1,205) | 0.034* |
| Male sex | 136 (54.6) | 45 (50.6) | 69 (54.8) | 22 (64.7) | 0.370 | 42 (46.2) | 17 (42.5) | 21 (48.8) | 4 (50.0) | 0.818 |
| CS | 125 (50.2) | 41 (46.1) | 69 (54.8) | 15 (44.1) | 0.340 | 84 (92.3) | 37 (92.5) | 39 (90.7) | 8 (100.0) | 1.000 |
| Short cervix | 45 (18.1) | 11 (12.4) | 25 (19.8) | 9 (26.5) | 0.146 | 7 (7.7) | 1 (2.5) | 5 (11.6) | 1 (12.5) | 0.247 |
| GDM | 15 (6.0) | 3 (3.4) | 9 (7.1) | 3 (8.8) | 0.398 | 5 (5.5) | 2 (5.0) | 2 (4.7) | 1 (12.5) | 0.601 |
| PIH | 39 (15.7) | 20 (22.5) | 15 (11.9) | 4 (11.8) | 0.088 | 56 (61.5) | 24 (60.0) | 27 (62.8) | 5 (62.5) | 0.948 |
| HCA | 107 (43.3) | 34 (39.1) | 61 (48.4) | 12 (35.3) | 0.239 | 21 (23.1) | 8 (20.0) | 11 (25.6) | 2 (25.0) | 0.757 |
| Oligohydramnios | 31 (12.4) | 10 (11.2) | 17 (13.5) | 4 (11.8) | 0.929 | 23 (25.3) | 7 (17.5) | 13 (30.2) | 3 (37.5) | 0.300 |
| Fetal distress | 26 (10.4) | 6 (6.7) | 14 (11.1) | 6 (17.6) | 0.190 | 61 (67.0) | 29 (72.5) | 27 (62.8) | 5 (62.5) | 0.635 |
| Uncontrolled preterm labor | 158 (63.5) | 53 (59.6) | 88 (69.8) | 17 (50.0) | 0.065 | 16 (17.6) | 8 (20.0) | 6 (14.0) | 2 (25.0) | 0.599 |
| Cord pH | 7.30 (7.26–7.35) | 7.31 (7.27–7.35) | 7.30 (7.25–7.34) | 7.29 (7.25–7.32) | 0.130 | 7.24 (7.18–7.29) | 7.26 (7.21–7.29) | 7.22 (7.16–7.28) | 7.24 (7.18–7.31) | 0.512 |
| 1-min AS | 5 (3–7) | 6 (4–7) | 5 (3–7) | 5 (2–7) | 0.135 | 4 (3–6) | 4 (3–6) | 4 (2–5) | 4 (3–6) | 0.423 |
| 5-min AS | 7 (6–8) | 7 (6–8) | 7 (6–8) | 7 (5–8) | 0.178 | 7 (6–7) | 7 (6–8) | 7 (5–7) | 7 (6–8) | 0.024* |
2. Mortality and in-hospital outcome
Table 2.
| Characteristic |
AGA without IUGR |
IUGR |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=249) | Complete ACS (n=89, 35.7%) | Incomplete ACS (n=126, 50.6%) | No ACS (n=34, 13.7%) | P-value | Total (n=91) | Complete ACS (n=40, 43.9%) | Incomplete ACS (n=43, 47.3%) | No ACS (n=8, 8.8%) | P-value | |
| Death | 11 (4.4) | 2 (2.2) | 5 (4.0) | 4 (11.8) | 0.072 | 11 (12.1) | 2 (5.0) | 7 (16.3) | 2 (25.0) | 0.119 |
| RDS | 63 (25.3) | 18 (20.2) | 32 (25.4) | 13 (38.2) | 0.121 | 25 (27.5) | 11 (27.5) | 11 (25.6) | 3 (37.5) | 0.828 |
| Surfactant use | 76 (30.5) | 22 (24.7) | 40 (31.7) | 14 (41.2) | 0.190 | 36 (39.6) | 12 (30.0) | 20 (46.5) | 4 (50.0) | 0.242 |
| Prophy S | 21 (8.4) | 5 (5.6) | 14 (11.1) | 2 (5.9) | 0.353 | 14 (15.4) | 3 (7.5) | 10 (23.3) | 1 (12.5) | 0.109 |
| Rescue S | 60 (24.1) | 18 (20.2) | 30 (23.8) | 12 (35.3) | 0.216 | 24 (26.4) | 10 (25.0) | 11 (25.6) | 3 (37.5) | 0.732 |
| Surfactant multiple | 11 (4.4) | 3 (3.4) | 6 (4.8) | 2 (5.9) | 0.690 | 5 (5.5) | 3 (7.5) | 2 (4.7) | 0 (0.0) | 0.793 |
| PDA with treatment | 88 (35.3) | 30 (33.7) | 43 (34.1) | 15 (44.1) | 0.514 | 46 (50.5) | 21 (52.5) | 21 (48.8) | 4 (50.0) | 0.951 |
| Pharmacological | 80 (32.1) | 28 (31.5) | 39 (31.0) | 13 (38.2) | 0.712 | 43 (47.3) | 19 (47.5) | 21 (48.8) | 3 (37.5) | 0.905 |
| Surgical | 27 (10.8) | 10 (11.2) | 11 (8.7) | 6 (17.6) | 0.337 | 9 (9.9) | 3 (7.5) | 5 (11.6) | 1 (12.5) | 0.669 |
| Sepsis | 40 (16.1) | 17 (19.1) | 18 (14.3) | 5 (14.7) | 0.622 | 21 (23.1) | 7 (17.5) | 11 (25.6) | 3 (37.5) | 0.396 |
| Early | 12 (4.8) | 5 (5.6) | 6 (5.6) | 0 (0.0) | 0.503 | 3 (3.3) | 0 (0.0) | 3 (7.0) | 0 (0.0) | 0.313 |
| Late | 33 (13.3) | 13 (14.6) | 15 (11.9) | 5 (14.7) | 0.839 | 19 (20.9) | 7 (17.5) | 9 (20.9) | 3 (37.5) | 0.439 |
| NEC ≥stage 2b | 7 (2.8) | 4 (4.5) | 2 (1.6) | 1 (2.9) | 0.359 | 6 (6.6) | 1 (2.5) | 4 (9.3) | 1 (12.5) | 0.326 |
| IVH ≥grade 3 | 12 (4.8) | 5 (5.6) | 5 (4.0) | 2 (5.9) | 0.772 | 5 (5.5) | 1 (2.5) | 4 (9.3) | 0 (0.0) | 0.480 |
| Hypotension | 27 (10.8) | 6 (6.7) | 12 (9.5) | 9 (26.5) | 0.012* | 15 (16.5) | 3 (7.5) | 10 (23.3) | 2 (25.0) | 0.089 |
| iNO use within 14 d | 17 (6.8) | 3 (3.4) | 11 (8.7) | 3 (8.8) | 0.262 | 9 (9.9) | 3 (7.5) | 3 (7.0) | 3 (37.5) | 0.069 |
| AI-HCS | 11 (4.4) | 2 (2.2) | 7 (5.6) | 2 (5.9) | 0.434 | 4 (4.4) | 1 (2.5) | 3 (7.0) | 0 (0.0) | 0.737 |
| Moderate to severe BPD | 38 (15.9) | 14 (16.1) | 18 (14.8) | 6 (20.0) | 0.750 | 26 (31.3) | 10 (26.3) | 13 (34.2) | 3 (42.9) | 0.564 |
| Severe BPD | 16 (6.7) | 4 (4.6) | 8 (6.6) | 4 (13.3) | 0.255 | 13 (15.7) | 5 (13.2) | 6 (15.8) | 2 (28.6) | 0.600 |
| ROP requiring treatment | 15 (6.3) | 4 (4.6) | 7 (5.7) | 4 (13.3) | 0.211 | 5 (5.8) | 1 (2.6) | 4 (10.0) | 0 (0.0) | 0.486 |
| Discharge with respiratory support | 38 (15.3) | 14 (15.7) | 18 (14.3) | 6 (17.6) | 0.879 | 19 (20.9) | 7 (17.5) | 11 (25.6) | 1 (12.5) | 0.630 |
| Hospital day | 36 (18–69) | 33 (18–70) | 39 (19–68) | 31 (14–63) | 0.608 | 59 (35–81) | 54 (32–76) | 66 (39–88) | 50 (33–69) | 0.325 |
| Hospital day in survivors | 38 (20–69) | 33 (20–71) | 39 (20–70) | 36 (16–73) | 0.838 | 62 (43–88) | 55 (37–80) | 70 (53–93) | 55 (34–79) | 0.081 |
Values are expressed as number (%) or median (range). Chi-square test, Fisher’s exact test, or Kruskal-Wallis test.
Abbreviations: ACS, antenatal corticosteroid use; AGA, appropriate for gestational age; IUGR, intrauterine growth restriction; RDS, respiratory distress syndrome; Prophy S, prophylactic surfactant use; Rescue S, rescue surfactant use; PDA, patent ductus arteriosus; NEC, necrotizing enterocolitis; IVH, intraventricular hemorrhage; iNO, inhaled nitric oxide; AI-HCS, transient adrenal insufficiency with hydrocortisone use; BPD, bronchopulmonary dysplasia; ROP, retinopathy of prematurity.
Table 3.
| Variable |
AGA without IUGR* |
IUGR† |
||||||
|---|---|---|---|---|---|---|---|---|
|
Complete courses of ACS use |
Incomplete courses of ACS use |
Complete courses of ACS use |
Incomplete courses of ACS use |
|||||
| aOR (95% CI) | P-value | aOR (95% CI) | P-value | aOR (95% CI) | P-value | aOR (95% CI) | P-value | |
| Primary outcomes | ||||||||
| Death | 0.13 (0.02–0.78) | 0.026‡ | 0.18 (0.04–0.78) | 0.021‡ | 0.06 (0.00–1.07) | 0.055 | 0.11 (0.01–1.61) | 0.107 |
| RDS | 0.44 (0.18–1.05) | 0.063 | 0.58 (0.26–1.31) | 0.189 | 0.39 (0.06–2.40) | 0.309 | 0.16 (0.02–1.08) | 0.060 |
| Surfactant use | 0.49 (0.21–1.13) | 0.094 | 0.68 (0.31–1.49) | 0.331 | 0.19 (0.02–1.94) | 0.162 | 0.14 (0.01–1.47) | 0.102 |
| Prophylactic surfactant use | 0.84 (0.15–4.63) | 0.837 | 1.64 (0.35–7.75) | 0.533 | 0.41 (0.03–5.38) | 0.497 | 1.02 (0.10–10.92) | 0.987 |
| Rescue surfactant use | 0.50 (0.21–1.21) | 0.126 | 0.62 (0.27–1.41) | 0.255 | 0.39 (0.06–2.40) | 0.309 | 0.16 (0.02–1.08) | 0.060 |
| Secondary outcomes | ||||||||
| PDA treatment | 0.60 (0.27–1.37) | 0.226 | 0.63 (0.29–1.38) | 0.249 | 1.17 (0.24–5.73) | 0.845 | 0.78 (0.16–3.92) | 0.762 |
| IVH ≥grade 3 | 0.80 (0.14–4.46) | 0.799 | 0.54 (0.10–2.99) | 0.482 | 0.54 (0.02–82.19) | 0.726 | 1.12 (0.09–159.48) | 0.941 |
| NEC ≥stage 2b | 1.90 (0.20–18.30) | 0.580 | 0.67 (0.06–7.94) | 0.752 | 0.11 (0.01–2.59) | 0.172 | 0.28 (0.02–3.87) | 0.344 |
| Sepsis | 1.32 (0.44–3.97) | 0.619 | 0.88 (0.30–2.60) | 0.814 | 0.22 (0.03–1.63) | 0.138 | 0.19 (0.03–1.43) | 0.106 |
| iNO within 14 d | 0.41 (0.08–2.15) | 0.289 | 1.05 (0.27–4.12) | 0.944 | 0.07 (0.01–0.67) | 0.020‡ | 0.04 (0.01–0.37) | 0.005‡ |
| Hypotension within 7 d | 0.20 (0.06–0.64) | 0.006‡ | 0.26 (0.09–0.70) | 0.008‡ | 0.08 (0.00–1.57) | 0.096 | 0.16 (0.01–2.70) | 0.205 |
| Moderate to severe BPD or death | 0.51 (0.20–1.30) | 0.159 | 0.45 (0.18–1.11) | 0.083 | 0.18 (0.02–1.75) | 0.140 | 0.12 (0.01–1.21) | 0.071 |
| Severe BPD or death | 0.24 (0.07–0.77) | 0.016‡ | 0.33 (0.12–0.92) | 0.035‡ | 0.16 (0.02–1.55) | 0.113 | 0.12 (0.01–1.19) | 0.069 |
Abbreviations: ACS, antenatal corticosteroid; AGA, appropriate for gestational age; IUGR, intrauterine growth restriction; aOR, adjusted odds ratio; CI, confidence intervals; RDS, respiratory distress syndrome; PDA, patent ductus arteriosus; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; iNO, inhaled nitric oxide; BPD, bronchopulmonary dysplasia.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download