Abstract
Hyponatremia is defined as a plasma sodium concentration of <135 mEq/L. It is a common electrolyte imbalance in newborns. We report the case of a term neonate with cleft lip, cleft palate, imperforate anus, normal male karyotype, and chronic hyponatremia. On the 4th day of life, he showed hyponatremia (plasma sodium concentration 130 mEq/L) with low serum osmolality (275 mOsm/kg), high urine sodium (116.7 mEq/L), and high urine osmolality (412 mOsm/kg). His thyroid and adrenal functions were normal. Despite intravenous and oral sodium supplementation and hydrocortisone treatment, hyponatremia persisted. Brain magnetic resonance imaging showed normal results. He was diagnosed as having reset osmostat, a rare subtype of the syndrome of inappropriate secretion of antidiuretic hormone characterized by a subnormal threshold for antidiuretic hormone secretion, with hypotonic hyponatremia.
Hyponatremia is one of the most common electrolyte disturbances, reported in up to 25% to 65% of the ill newborns in the neonatal intensive care units [1]. It is defined as a plasma sodium concentration of <135 mEq/L [2]. Hyponatremia in neonates usually develops owing to the inability of the immature neonatal kidney to excrete excess water. Hypotonic hyponatremia with a serum osmolality of <280 mOsm/kg is relatively common in newborns. It can be clinically classified into three types according to the extracellular volume status. Hypovolemic hyponatremia occurs when the sodium loss exceeds the water loss. Renal loss or extrarenal loss (e.g., gastrointestinal loss, 3rd space loss, and skin loss) of sodium can cause this condition. Hypervolemic hyponatremia is caused by water accumulation in excess of the sodium intake. Euvolemic hyponatremia occurs secondary to hypothyroidism, glucocorticoid deficiency, or syndrome of inappropriate antidiuretic hormone (SIADH). About 60% of the patients with chronic euvolemic hyponatremia have SIADH, which is the most common cause of euvolemic hyponatremia [2]. In 1967, Bartter and Schwartz [3] described the cardinal criteria of SIADH, as follows: (1) hyponatremia with hypo-osmolality of the serum and extracellular fluid; (2) continuous sodium excretion by the kidney; (3) absence of volume depletion, meaning normal skin turgor and blood pressure; (4) urine osmolality greater than plasma osmolality, meaning inappropriate urine concentration; (5) normal renal function; and (6) normal adrenal function. An elevated concentration of plasma arginine vasopressin (AVP), one of the main antidiuretic hormones (ADHs), is essential in the development of SIADH, independent of primary or secondary elevation. The measurement of plasma AVP concentration is not helpful in diagnosing SIADH because many other conditions of hyponatremia are associated with the secondary elevation of AVP concentration [2].
In most cases, SIADH in neonates is caused by central nervous system infection and brain injury; however, in some rare cases, SIADH may be caused by reset osmostat. The diagnostic criteria for reset osmostat include euvolemia, normal renal, adrenal, and thyroid functions, ability to concentrate the urine and excrete the overloaded fluid, and hyponatremia despite salt overload [4]. Here, we report a case of a newborn with refractory hyponatremia secondary to reset osmostat, a rare type of SIADH.
A male infant was born as small for gestational age at 37 weeks and 4 days of gestation via a vaginal delivery. Both his parents were 24 years old. He was the 5th child of his mother (of Korean ethnicity) and has no parental consanguinity. He was the 1st baby of his father. His mother was phenotypically normal. His father had midface hypoplasia. His father had undergone a correction operation for cleft lip and palate when he was young. His mother had a history of smoking five cigarettes per day. She did not take any drug during the pregnancy. Prenatal ultrasonography detected bilateral hydronephrosis and facial dysmorphism including a flat face, cleft lip, and cleft palate with normal fetal movement.
The patient’s Apgar scores were 8 and 9 at 1 and 5 minutes, respectively. He weighed 2,500 g (3rd to 10th percentile) and was 47 cm long (10th to 25th percentile) with a head circumference of 30.5 cm (3rd to 10th percentile). He had brachycephaly, a short anteroposterior diameter, midface hypoplasia with hypertelorism, small nose, low nasal bridge, flat occiput, maxillary hypoplasia, cleft lip, cleft palate (Figure 1), and low-type imperforate anus with fistula (Figure 2). Echocardiography showed a patent ductus arteriosus and an atrial septal defect. Sacral ultrasound showed normal results. Renal ultrasound revealed bilateral hydronephrosis.
On the 5th day of life, the baby underwent an anoplasty. He passed normal stool within 24 hours after the operation. He was able to take milk. His plasma sodium concentration started to decrease from the 4th day of life. Thereafter, he had hyponatremia with sodium concentration maintained at 130 mEq/L. It was neither caused by volume overloading during anoplasty nor by the inability of the immature kidney to excrete free water. He showed low plasma sodium concentration (125 mEq/L), low serum osmolality (275 mOsm/kg), high urine sodium concentration (116.7 mEq/L), and high urine osmolality (412 mOsm/kg). Further tests performed, including glucose, blood urea nitrogen, creatinine, uric acid, and urinalysis, showed normal results. Endocrinologic assessments for adrenocorticotropic hormone, cortisol, aldosterone, parathyroid hormone, triiodothyronine, free thyroxine, and thyroid-stimulating hormone also yielded normal findings. Brain magnetic resonance imaging showed normal pituitary gland and hypothalamus. Chromosomal analysis showed normal male karyotype, after obtaining informed consent.
Daily intravenous sodium replacement (5 to 8 mEq/kg) was given from the 5th postnatal day. However, hyponatremia persisted. Hydrocortisone (1 mg/kg) was injected daily from the 13th postnatal day until adrenal insufficiency was excluded. However, hyponatremia persisted. Intravenous hypertonic sodium replacement (6 mEq/kg) was administered from the 19th postnatal day (Table 1). Despite sodium replacement and hydrocortisone treat ment with volume restriction to 120 mL/kg, his sodium concentration remained low, between 125 and 128 mEq/L. We started oral supplementation of sodium from 2.5 to 5 mEq/kg from the 25th postnatal day. His sodium concentration increased to 131 mEq/L, which was still low. However, it did not decrease to <128 mEq/L. We added sodium in the milk four times per day. Treatment with furosemide from the 40th postnatal day to induce excretion of free water was not effective in increasing his sodium concentration. Thus, we stopped furosemide on the 60th postnatal day at the outpatient clinic. His plasma sodium concentration ranged from 128 to 131 mEq/L and serum osmolality was 267 to 272 mOsm/kg. His urine sodium concentration was 18 to 75 mEq/L and urine osmolality was 127 to 246 mOsm/kg. Both urine sodium concentration and urine osmolality increased after the oral supplementation of sodium. He was fed well, with the use of a Haberman feeder because of his cleft lip and cleft palate. His weight gain was good after supplementation with sodium and sufficient calories (>90 kcal/kg) after the 7th day of birth. He was discharged from the neonatal intensive care unit on the 54th day of life with a sodium concentration of 131 mEq/L. He was clinically well with normal growth and development, including head control, pursuit eye movements, babbling, and smiling in response to face and voice. He did not show any kind of seizures due to hyponatremia. We continued to supply oral sodium (5 mEq/kg) and monitor his plasma sodium concentration, growth, and development.
Hyponatremia is believed to be a benign disease; however, it is a very common condition in newborn babies. Euvolemic hyponatremia usually occurs secondary to hypothyroidism, glucocorticoid deficiency, or increased ADH concentration [5]. Sodium is a crucial element for growth and weight gain. It is present in the bone, cartilage, and connective tissues. Sodium is also essential for the development of the brain and the central nervous system. Researchers have reported that preterm infants with chronic hyponatremia experience delayed development and poor growth [1,4,6-8]. Preterm infants with chronic hyponatremia associated with sodium-restricted diets show impaired development of body physique and poor neurodevelopmental outcomes at school ages, compared with preterm infants with normal sodium concentration associated with sodium-supplemented diets [7].
Reset osmostat is one of the four subtypes of SIADH, based on ADH secretion. Type A is characterized by high and erratic fluctuation in the concentration of ADH that is not physiologically affected by serum osmolality. Type B is defined as modest and constant leakage of ADH. Type C is defined as reset osmostatinduced excretion of ADH caused by a decrease in the plasma osmolality threshold. Plasma sodium adjusted to a lower concentration between 125 and 135 mEq/L. Type D is characterized by normal osmoregulation of ADH with upregulation of vasopressin receptor 2 expression without ADH elevation [4]. According to the diagnostic criteria, normal renal, adrenal, and thyroid functions, normal capacity to concentrate urine and excrete overloaded fluid, and hyponatremia despite salt replacement should be present to diagnose reset osmostat euvolemia [4].
Babies who experience fetal distress, a difficult delivery, or perinatal/neonatal asphyxia secondarily develop elevated circulating ADH concentrations, compared with babies born uneventfully. However, true SIADH with primary elevation of ADH is relatively rare in the newborn period. The diagnosis of SIADH should be made according to the diagnostic criteria, including normal blood pressure and normal adrenal, thyroid, renal, and cardiac functions [9]. In most cases, the duration of hyponatremia caused by SIADH is <3 days. Chronic SIADH persisting for >3 days is rare in children, and only 15 cases have been reported. The incidence of SIADH in newborns is unknown owing to its extreme rarity [5].
Some rare cases of SIADH caused by reset osmostat have been reported in newborns. A case of reset osmostat in a male preterm infant (36 weeks of gestational age, with cleft lip and palate at prenatal ultrasonography) has been reported. The infant showed hyponatremia (plasma sodium concentration 131 mEq/L) on the 7th day of life [4]. Despite fludrocortisone and oral sodium replacement, his plasma sodium concentration was maintained between 124 and 130 mEq/L. At 16 to 19 months of age, he showed progressive growth restriction and mild delay in development [4]. Another case of reset osmostat was reported in a female preterm infant at 36 weeks of gestational age, with cleft lip and cleft palate at prenatal ultrasonography, showing panhypopituitarism with normal brain structure and hyponatremia (plasma sodium concentration between 124 and 130 mEq/L) from the 1st postnatal day [6]. Her hyponatremia persisted despite replacement of hydrocortisone with fludrocortisone and oral sodium. At 15 months of age, she showed growth restriction with mild delay in development [6]. A case of reset osmostat was also reported in a female term infant with a dysmorphic face. She presented with midface hypoplasia with hypertelorism, small nose and mouth, choanal atresia, maxillary hypoplasia, and high-arched palate. She showed a chromosomal abnormality with unbalanced translocation between chromosome 13 and chromosome X, resulting in 46, X, -X, +der(X) t(X; 13) (p22.1; q22). Hyponatremia persisted (plasma sodium concentration between 127 and 133 mEq/L) despite intravenous/oral sodium replacement and glucocorticoid therapy. At 7 months of age, she showed growth restriction with diminished growth velocity and delayed development, including delayed language, motor, and cognitive functions [8].
There is no clear consensus about the treatment of hyponatremia. The treatment and outcomes have been variable. Thus, treatment varies from water restriction to administration of demeclocycline, lithium, furosemide, and AVP antagonists, such as urea, for observation without any treatment [10]. In our case, intravenous sodium supplementation and hydrocortisone treatment were not effective; after treatment, the plasma sodium concentration ranged between 127 and 129 mEq/L. Furosemide causes overflow to the concentrating areas of the nephron, which overwhelms the normal transport capacity independent of the baseline water permeability, thus, inducing free water clearance. Thus, we started to use furosemide. Oral supplementation with sodium and oral furosemide treatment were continued at the outpatient department. Since the plasma sodium concentration was between 128 and 131 mEq/L despite oral sodium and furoemide treatment, furosemide was stopped. Furosemide and hydrocortisone treatment did not lower the baseline serum sodium concentration compared with oral sodium supplementation only, and furosemide did not increase the urine sodium concentration.
There have been some reported cases of reset osmostat in newborn babies with cleft lip and palate, or a high-arched palate. Thus, clinicians who treat babies with chronic hyponatremia should suspect reset osmostat especially in babies with cleft lip and palate or a high-arched palate. Patients with chronic hyponatremia caused by reset osmostat need specific treatment, including sodium replacement to prevent developmental delay and growth restriction.
REFERENCES
1. Marcialis MA, Dessi A, Pintus MC, Irmesi R, Fanos V. Neonatal hyponatremia: differential diagnosis and treatment. J Matern Fetal Neonatal Med. 2011; 24 Suppl 1:75–9.
2. Baylis PH. The syndrome of inappropriate antidiuretic hormone secretion. Int J Biochem Cell Biol. 2003; 35:1495–9.
3. Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967; 42:790–806.
4. Vale BM, Morais S, Mesquita J, Mimoso G. Reset osmostat: a rare cause of hyponatraemia. BMJ Case Rep. 2015; 2015:bcr2013009111.
5. Marcialis MA, Dessi A, Contu S, Fanos V. Nephrogenic syndrome of inappropriate antidiuresis: a novel cause of euvolemic hypotonic hyponatremia in newborns. Diagnosis and practical management. J Matern Fetal Neonatal Med. 2009; 22 Suppl 3:67–71.
6. Thiagarajan R, La Gamma E, Dey S, Blethen S, Wilson TA. Hyponatremia caused by a reset osmostat in a neonate with cleft lip and palate and panhypopituitarism. J Pediatr. 1996; 128:561–3.
7. Moritz ML, Ayus JC. Hyponatremia in preterm neonates: not a benign condition. Pediatrics. 2009; 124:e1014. –6.
8. Gupta P, Mick G, Fong CT, Jospe N, McCormick K. Hyponatremia secondary to reset osmostat in a child with a central nervous system midline defect and a chromosomal abnormality. J Pediatr Endocrinol Metab. 2000; 13:1637–41.
9. Modi N. Hyponatraemia in the newborn. Arch Dis Child Fetal Neonatal Ed. 1998; 78:F81–4.
10. Chehade H, Rosato L, Girardin E, Cachat F. Inappropriate antidiuretic hormone secretion: long-term successful urea treatment. Acta Paediatr. 2012; 101:e39–42.
Figure 1.
Photographs of the face. (A) Hypertelorism, maxillary hypoplasia, low nasal bridge, and cleft lip and palate. (B, C) Short anteroposterior diameter with a flat occiput.
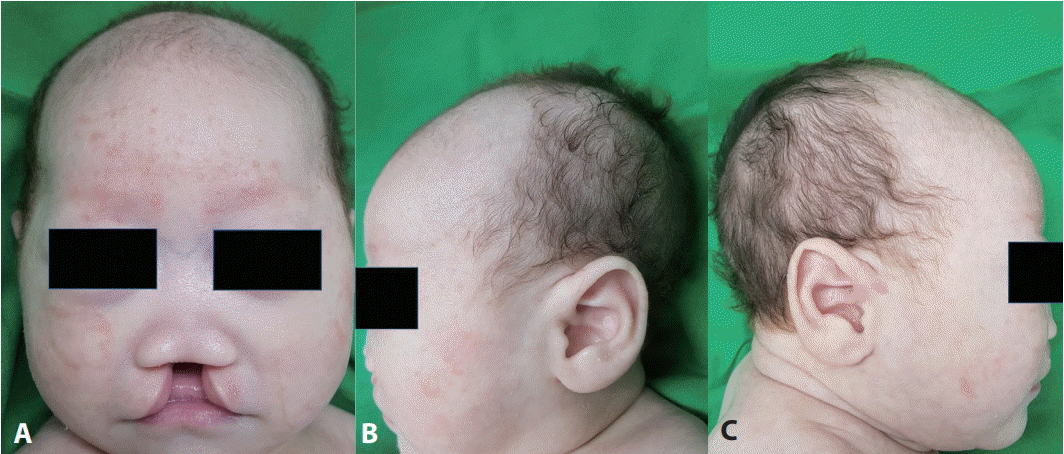
Table 1.
Daily Weight, Input, Output, Sodium Concentration, and Treatment
| Postnatal day (day) | Weight (kg) | Input (mL/kg/day) | Urine output (mL/kg/hr) | Serum Na (mEq/L) | Serum osmolality (mOsm/kg) | Urine Na (mEq/L) | Urine osmolality (mOsm/kg) | Treatment | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.50 | 60 | 0.5 | 139 | |||||
| 4 | 2.52 | 71 | 2.7 | 130 | Na replace (2 mEq/kg, iv) | ||||
| 5 | 2.42 | 110 | 2.5 | 127 | Na replace (5 mEq/kg, iv) Anoplasty | ||||
| 8 | 2.56 | 130 | 3.5 | 127 | Na replace (8 mEq/kg, iv) | ||||
| 13* | 2.65 | 130 | 3.2 | 125 | 412 | 116.7 | 275 | Hydrocortisone (1 mg/kg) | |
| 19 | 2.65 | 124 | 2.6 | 127 | 275 | Hydrocortisone stop Hypertonic saline (6 mEq/kg, iv) | |||
| 20 | 2.70 | 115 | 3.3 | 128 | Hypertonic saline (6mEq/kg, iv) | ||||
| 22 | 2.71 | 115 | 2.8 | 129 | 161 | 54 | 267 | Hypertonic saline (6 mEq/kg, iv) | |
| 25 | 2.66 | 120 | 3.2 | 127 | Na replace (2.5 mEq/kg, oral) | ||||
| 29 | 2.75 | 145 | 3.0 | 131 | 127 | 18.2 | 271 | Na replace (2.5 mEq/kg, oral) | |
| 34 | 2.96 | 164 | 3.8 | 131 | Na replace (5 mEq/kg, oral) | ||||
| 40† | 3.26 | 156 | 4.2 | 128 | 246 | 33 | 269 | Furosemide (2 mg/kg) Na replace (5 mEq/kg, oral) | |
| 48 | 3.58 | 150 | 3.2 | 131 | 229 | 75.3 | 272 | Furosemide (2 mg/kg) Na replace (5 mEq/kg, oral) | |
| 60 | 4.10 | 128 | Furosemide stop Na replace (5 mEq/kg, oral) |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download