INTRODUCTION
Cardiac resynchronization therapy (CRT) using multisite ventricular pacing has been widely considered a beneficial option for treating adult patients with heart failure who do not improve after medical treatment. In adults, current guidelines recommend CRT as a class IA indication in patients with symptomatic heart failure, low ejection fraction (EF ≤35%), left bundle branch block (LBBB), and wide QRS duration (≥150 ms) despite optimal medical therapy.
1) Multiple randomized controlled studies have demonstrated that CRT leads to reverse ventricular remodeling, improvement of ventricular function and functional class, and a decrease in mortality and hospitalization due to heart failure.
2)3)
Although clinical evidence supports CRT’s effectiveness in adults with heart failure, its efficacy in pediatric and older patients with congenital heart disease (CHD) has not been well established. Thus, the CRT criteria were mainly drawn from the adult guidelines. However, pediatric patients are diverse in age and body size. Moreover, this group is heterogeneous in terms of cardiac anomaly, ventricular anatomy, conduction system, and cardiac surgery history. Hence, performing prospective and randomized trials in children and patients with CHD is challenging. Furthermore, there are few case reports on pediatric CRT applications in South Korea.
This study aimed to evaluate early and late outcomes of CRT and technical issues associated with CRT application in patients with CHD or those aged ≤18 years.
METHODS
Ethical statement
The study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. 2107-193-1237), and the requirement for written informed consent was waived owing to the study’s retrospective nature. This study complied with the principles of the Declaration of Helsinki.
Study population
Patients under 18 or with CHD who underwent CRT implantation at a single tertiary center between January 2008 and May 2020 were enrolled. This was a retrospective review of available medical records, including underlying heart disease, echocardiography, electrocardiography, chest radiography, and clinical data. Patients were subcategorized by cardiac problems such as congenital complete atrioventricular (AV) block in a structurally normal heart, dilated cardiomyopathy (DCM) with LBBB, and CHD. CRT implantation was decided case-by-case, considering both electrical and mechanical dyssynchrony. Furthermore, indications in each patient with heart failure were as follows: right ventricle (RV) free wall pacing-induced cardiomyopathy in a patient with congenital complete AV block, DCM with LBBB, CHD with wide QRS duration, and mechanical dyssynchrony based on echocardiography or pacing-induced cardiomyopathy.
The response to CRT was evaluated using ventricular function by EF and biplane method, QRS duration in electrocardiography, left ventricular end-diastolic dimension (LVEDD), and New York Heart Association (NYHA) functional class or modified Ross classification.
4) LVEDD was measured using the z-score in 12 patients with systemic left ventricle (LV). Patients with CHD with systemic RV or a functional single ventricle were excluded from the analysis of ventricular dimension changes. Responders were defined as those with an increase in systemic ventricular EF >10% or an improvement >1 NYHA or modified Ross functional class.
5) CRT responses were analyzed using data before CRT, 1 year after CRT (defined as early outcome), 4 to 5 years after CRT (defined as late outcome). Late outcomes were available in 8 of the 16 patients.
Statistical analysis
Values are expressed as median (range) or mean (±standard deviation), according to the mode of data distribution. Categorical variables were evaluated using chi-square analysis. Paired comparisons of continuous variables were performed using Wilcoxon signed-rank test. All statistical analyses were performed using SPSS software (version 23.0; SPSS Inc., Armonk, NY, USA), and statistical tests were considered significant when p value <0.05.
RESULTS
Demographics
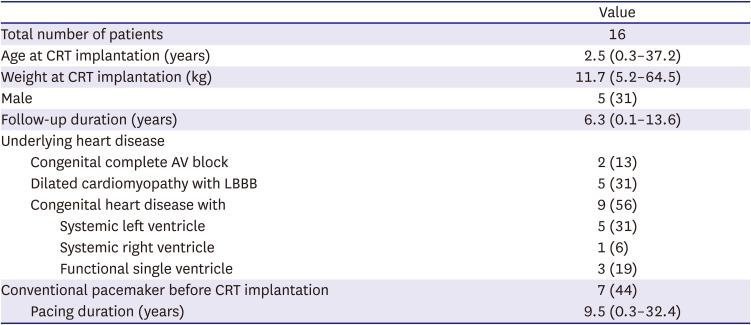
CRT devices were implanted in 16 patients (
Table 1,
Supplementary Table 1). This study consisted of 5 males (31%) and 11 females (69%). The median follow-up duration was 6.3 years (range, 0.1 to 13.6 years). Patients were categorized into 3 groups: 2 (13%) had congenital complete AV block with previous RV pacing, 5 (31%) had DCM with LBBB, both in the group without CHD, and 9 (56%) had CHD. Among the patients with CHD, 5 (31%) had systemic LV, 1 (6%) had systemic RV, and 3 (19%) had an RV-type single ventricle.
Table 1
Demographic data
|
Value |
|
Total number of patients |
16 |
|
Age at CRT implantation (years) |
2.5 (0.3–37.2) |
|
Weight at CRT implantation (kg) |
11.7 (5.2–64.5) |
|
Male |
5 (31) |
|
Follow-up duration (years) |
6.3 (0.1–13.6) |
|
Underlying heart disease |
|
|
Congenital complete AV block |
2 (13) |
|
Dilated cardiomyopathy with LBBB |
5 (31) |
|
Congenital heart disease with |
9 (56) |
|
|
Systemic left ventricle |
5 (31) |
|
|
Systemic right ventricle |
1 (6) |
|
|
Functional single ventricle |
3 (19) |
|
Conventional pacemaker before CRT implantation |
7 (44) |
|
Pacing duration (years) |
9.5 (0.3–32.4) |

The median age at CRT implantation was 2.5 years (range, 3 months to 37.2 years). Of the 16 patients, conventional pacemakers had been implanted in 7 patients (44%) prior to CRT implantation because of congenital complete AV block in 2 and postsurgical heart block in 5. These patients had been paced for a median of 9.5 years (range, 3 months to 32.4 years) before upgrading to CRT.
CRT systems
CRT systems were placed non-transvenously by sternotomy using epicardial pacing leads in 13 patients (81.2%), and transvenously in 3 (18.8%). Except for 3 patients with the transvenous approach, patients with epicardial leads had their device pockets made in their subcostal space (n=3, 23%) or abdominal space (n=10, 77%). In one patient, the system was initially placed with an epicardial approach, which was changed to a transvenous approach because of extracardiac lead failure 8 years after the initial CRT. Twelve (75%) patients received CRT pacemakers, and 4 (25%) received CRT defibrillator (CRT with an implantable cardioverter-defibrillator).
Clinical outcomes
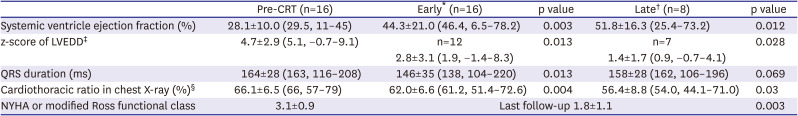
Table 2 summarizes the changes in quantitative measurements and clinical status before and after CRT. The early and late outcomes were respectively compared with pre-CRT data to evaluate the change from the baseline. The mean EF of the systemic ventricle before CRT was 28.1±10.0%, which significantly increased to 44.3±21.0% (p=0.003) in the early outcome and 51.8±16.3% (p=0.012) in late outcome groups (
Figure 1A,
Supplementary Figure 1). The mean z-score of LVEDD before CRT was 4.7±2.9, which decreased to 2.8±3.1 (p=0.013) in the early outcome and 1.4±1.7 (p=0.028) in the late outcome (
Figure 1B,
Supplementary Figure 2). Before receiving CRT, 7 patients had complete AV block congenitally or postoperatively, 6 had LBBB, and 3 had intraventricular conduction delays in the functional single ventricle. After CRT implantation, QRS duration tended to decrease from pre-CRT QRS duration of 164±28 ms to 146±35 ms (p=0.013) in the early outcome and to 158±28 ms in the late outcome (p=0.069), although the late outcome did not show statistical significance (
Figure 2). NYHA or modified Ross functional class improved significantly from 3.1±0.9 before CRT to 1.8±1.1 at the last follow-up (p=0.003) (
Figure 3).
Figure 1
Early and late changes of ventricular ejection fraction (A) and left ventricular end-diastolic dimension (B) before and after cardiac resynchronization therapy implantation.
cAVB = complete atrioventricular block; DCM = dilated cardiomyopathy; LBBB = left bundle branch block; LV = left ventricle.


Figure 2
Early and late changes of QRS duration before and after cardiac resynchronization therapy implantation.
cAVB = complete atrioventricular block; DCM = dilated cardiomyopathy; LBBB = left bundle branch block.
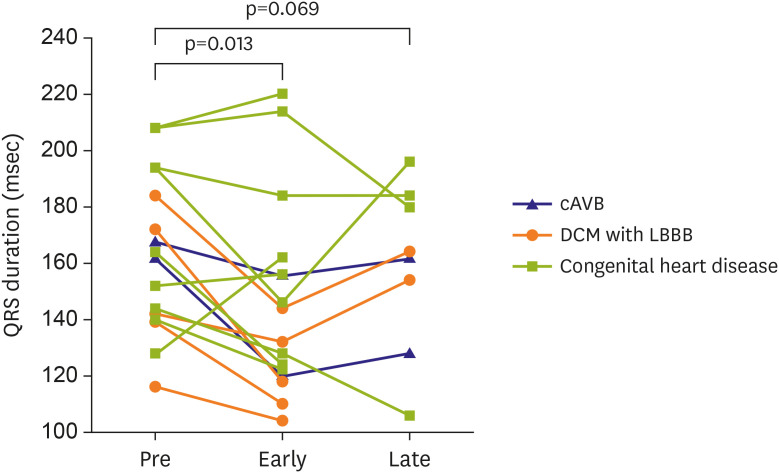

Figure 3
Early and late changes of New York Heart Association or modified Ross functional class.
cAVB = complete atrioventricular block; DCM = dilated cardiomyopathy; LBBB = left bundle branch block.


Table 2
Data analysis pre-CRT and after-CRT
|
Pre-CRT (n=16) |
Early* (n=16) |
p value |
Late† (n=8) |
p value |
|
Systemic ventricle ejection fraction (%) |
28.1±10.0 (29.5, 11–45) |
44.3±21.0 (46.4, 6.5–78.2) |
0.003 |
51.8±16.3 (25.4–73.2) |
0.012 |
|
z-score of LVEDD‡
|
4.7±2.9 (5.1, −0.7–9.1) |
n=12 |
0.013 |
n=7 |
0.028 |
|
2.8±3.1 (1.9, −1.4–8.3) |
1.4±1.7 (0.9, −0.7–4.1) |
|
QRS duration (ms) |
164±28 (163, 116–208) |
146±35 (138, 104–220) |
0.013 |
158±28 (162, 106–196) |
0.069 |
|
Cardiothoracic ratio in chest X-ray (%)§
|
66.1±6.5 (66, 57–79) |
62.0±6.6 (61.2, 51.4–72.6) |
0.004 |
56.4±8.8 (54.0, 44.1–71.0) |
0.03 |
|
NYHA or modified Ross functional class |
3.1±0.9 |
Last follow-up 1.8±1.1 |
0.003 |

CRT response according to subgroup
Twelve of the 16 (75%) patients were CRT responders. Proportion of responders differed between patients without CHD and those with CHD, although statistical significance was not reached (100% vs. 56%, p=0.088): 2/2 patients with congenital AV block (100%), 5/5 with DCM with LBBB (100%), and 5/9 with CHD (56%) were responders. CRT response did not depend on other factors such as type of systemic ventricle (83% in LV type vs. 50% in non-LV type, p=0.245), presence of LBBB (83% in LBBB vs. 70% in non-LBBB, p=1.0), mode of implantation (33% in transvenous vs. 85% in non-transvenous, p=0.136), and QRS duration before CRT implantation (162±26 ms in responder vs. 168±39 ms in non-responder, p=1.0) (
Supplementary Table 2). Both patients with pacemaker-induced cardiomyopathy showed normalized EF and ventricular diastolic dimensions after upgrading to CRT, which had been maintained for more than 10 years without medication (
Supplementary Table 3). In 5 patients with DCM and LBBB, 3 had more than 50% of EF and normal LV dimension, but 2 still had decreased EF (37–47%) and enlarged ventricular dimension (z-score 2.3–6.3), while clinical and functional improvement was maintained. All patients with DCM and LBBB had been on heart failure medications until the last follow-up (
Supplementary Table 3).
CRT in patients with CHD
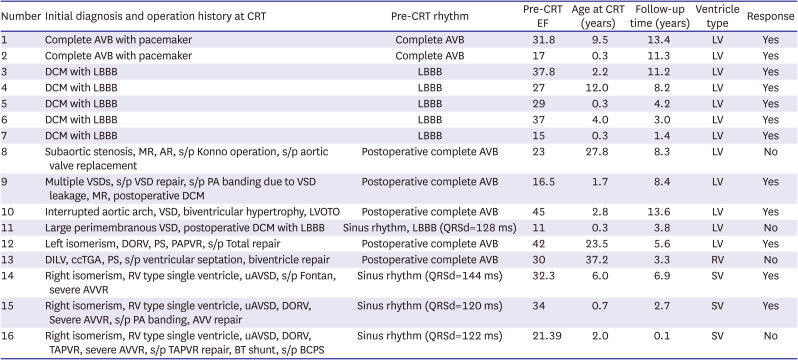
All 9 patients with CHD underwent heart surgery before CRT implantation (
Table 3). Five of the 9 CHD patients (56%) had postoperative complete AV block and were paced with a conventional pacemaker prior to CRT. Among the 5 patients with systemic LV, 3 showed responses to CRT, and 2 had no response and underwent heart transplantation. One patient with systemic RV who had undergone ventricular septation for double-inlet LV and ventriculoarterial discordance showed no response. No significant difference was observed in the CRT response between the LV- and non-LV-type systemic ventricles (3/5 [60%] vs. 2/4 [50%], p=1.0). We had 3 patients with a single ventricle who underwent CRT implantation. All patients had right isomerism, unbalanced AV septal defect, and severe AV valve regurgitation at the time of CRT implantation. Among them, 2 underwent CRT implantation and concomitant AV valve replacement. Both showed improvements in ventricular function, chamber dilatation, QRS duration, and functional class.
Table 3
Detailed information of patients
|
Number |
Initial diagnosis and operation history at CRT |
Pre-CRT rhythm |
Pre-CRT EF |
Age at CRT (years) |
Follow-up time (years) |
Ventricle type |
Response |
|
1 |
Complete AVB with pacemaker |
Complete AVB |
31.8 |
9.5 |
13.4 |
LV |
Yes |
|
2 |
Complete AVB with pacemaker |
Complete AVB |
17 |
0.3 |
11.3 |
LV |
Yes |
|
3 |
DCM with LBBB |
LBBB |
37.8 |
2.2 |
11.2 |
LV |
Yes |
|
4 |
DCM with LBBB |
LBBB |
27 |
12.0 |
8.2 |
LV |
Yes |
|
5 |
DCM with LBBB |
LBBB |
29 |
0.3 |
4.2 |
LV |
Yes |
|
6 |
DCM with LBBB |
LBBB |
37 |
4.0 |
3.0 |
LV |
Yes |
|
7 |
DCM with LBBB |
LBBB |
15 |
0.3 |
1.4 |
LV |
Yes |
|
8 |
Subaortic stenosis, MR, AR, s/p Konno operation, s/p aortic valve replacement |
Postoperative complete AVB |
23 |
27.8 |
8.3 |
LV |
No |
|
9 |
Multiple VSDs, s/p VSD repair, s/p PA banding due to VSD leakage, MR, postoperative DCM |
Postoperative complete AVB |
16.5 |
1.7 |
8.4 |
LV |
Yes |
|
10 |
Interrupted aortic arch, VSD, biventricular hypertrophy, LVOTO |
Postoperative complete AVB |
45 |
2.8 |
13.6 |
LV |
Yes |
|
11 |
Large perimembranous VSD, postoperative DCM with LBBB |
Sinus rhythm, LBBB (QRSd=128 ms) |
11 |
0.3 |
3.8 |
LV |
No |
|
12 |
Left isomerism, DORV, PS, PAPVR, s/p Total repair |
Postoperative complete AVB |
42 |
23.5 |
5.6 |
LV |
Yes |
|
13 |
DILV, ccTGA, PS, s/p ventricular septation, biventricle repair |
Postoperative complete AVB |
30 |
37.2 |
3.3 |
RV |
No |
|
14 |
Right isomerism, RV type single ventricle, uAVSD, s/p Fontan, severe AVVR |
Sinus rhythm (QRSd=144 ms) |
32.3 |
6.0 |
6.9 |
SV |
Yes |
|
15 |
Right isomerism, RV type single ventricle, uAVSD, DORV, Severe AVVR, s/p PA banding, AVV repair |
Sinus rhythm (QRSd=120 ms) |
34 |
0.7 |
2.7 |
SV |
Yes |
|
16 |
Right isomerism, RV type single ventricle, uAVSD, DORV, TAPVR, severe AVVR, s/p TAPVR repair, BT shunt, s/p BCPS |
Sinus rhythm (QRSd=122 ms) |
21.39 |
2.0 |
0.1 |
SV |
No |

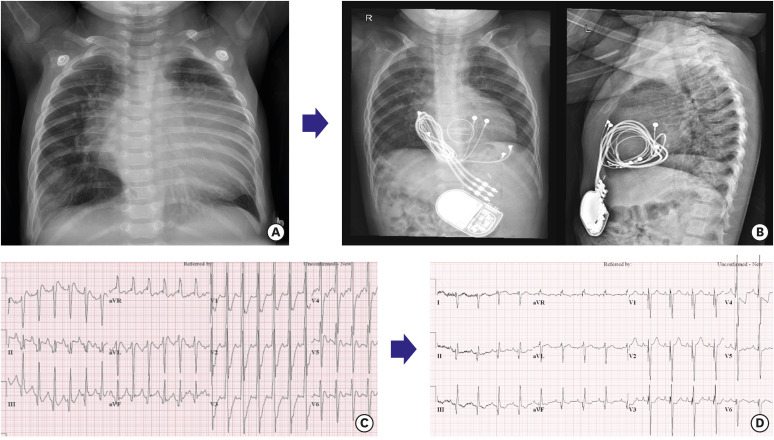
An 8-month-old male patient (patient 15,
Supplementary Table 1) had a complex cardiac anomaly with ventricular dysfunction: right isomerism, unbalanced AV septal defect, double outlet RV, functional single ventricle, and severe AV valve regurgitation. He underwent AV valve repair and pulmonary artery banding 7 days after birth and required postoperative support for extracorporeal membrane oxygenation for several days. He underwent redo-AV valve repair, due to severe AV valve regurgitation and ventricular dysfunction. However, ventricular dysfunction with severe valve regurgitation, mechanical dyssynchrony, and heart failure persisted with poor weight gain. At 6 months of age, AV valve replacement and CRT device implantation were performed. After the operation, cardiomegaly and ventricular dysfunction dramatically improved, as shown in
Figure 4 and
Supplementary Videos 1 and
2, and he underwent bilateral pulsatile bidirectional Glenn shunt at 22 months.
Figure 4
An 8-month-old boy with right isomerism, RV type single ventricle, severe atrioventricular valve underwent CRT implantation and concomitant atrioventricular valve replacement for severe ventricular dysfunction and valve regurgitation. Chest X-ray shows cardiomegaly before CRT (A), which improved 13 months after atrioventricular valve replacement and CRT implantation (B). The CRT ventricular leads were implanted at the apex of the ventricle and the RV outflow tract, respectively. Improvement of ventricular mechanical dyssynchrony and ventricular function in echocardiography after 3 months of CRT. The ejection fraction before CRT was 34%, which increased to 51% 3 months after CRT implantation and atrioventricular replacement. In addition, shortening of QRS duration from 162 ms to 108 ms supported the improvement of electrical dyssynchrony. (C) Before CRT; (D) After CRT.
CRT = cardiac resynchronization therapy; RV = right ventricle.

Complications
The incidence of acute complications within 3 months of CRT was 12.5% (2/16 patients). One patient had mediastinitis after epicardial CRT implantation, and one adult patient with CHD (patient 8,
Supplementary Table 1) had skin erosion and device exposure requiring system revision twice. Long-term complications occurred in 3 patients. Two patients had lead failure in one of the 2 ventricular leads, and one had infective endocarditis related to the pacemaker lead. No deaths were related to CRT device implantation.
At the latest follow-up, 15 of the 16 patients were alive, with 11 still receiving CRT. One death was secondary to multiorgan failure following heart failure early after CRT implantation surgery, and 2 non-responders underwent heart transplantation.
DISCUSSION
CRT is a powerful tool in patients with heart failure associated with LV dysfunction and intraventricular conduction delay. However, the current indication for CRT may not be easily translated to pediatric patients because the etiology of heart failure is usually different from that in adults. Despite the promotion of research on potential efficacy of CRT in pediatric and CHD patients with heart failure, studies on CRT performed in this group of patients are still lacking. Furthermore, the number of patients with CHD is growing in developed countries, and it is estimated that over 90% of children with CHD survive and reach adulthood with an improved life expectancy.
6) This increases the risk of lifelong complications, including heart failure.
7) Thus, CRT’s importance in pediatric and CHD patients has been increasing.
This report demonstrates the long-term outcomes of CRT in pediatric and CHD patients based on a single-center experience. CRT has resulted in positive outcomes in 75% of the total patients with the improvement of systemic ventricle function or NYHA functional class, which is compatible with previous studies.
8) We found that patients without CHD had better responses compared to patients with CHD, although statistical significance was not reached due to the small sample size. All patients with congenital complete AV block or DCM with LBBB responded. In particular, 2 patients with pacemaker-induced cardiomyopathy from RV pacing showed normalization of ventricular function and dimensions after upgrading the pacemaker to CRT, which is consistent with other pediatric and adult studies.
9)10) When reviewed, both patients underwent RV-free wall pacing before CRT implantation. In a previous study, we demonstrated that RV free-wall pacing was a significant risk factor for LV dysfunction in children with congenital heart block and epicardial pacing, and that ventricular lead relocation to the RV apex or CRT upgrade resulted in LV function improvement.
11)
DCM patients with LBBB also showed improved LV function and functional status in this study. However, Janousek et al.
9) reported that pediatric patients with primary cardiomyopathy showed a low response to CRT (responder 3/9, 33%), which was a risk factor for non-response to CRT (p=0.002) with NYHA class (p=0.003) in a multivariate analysis. They included patients with storage disease and a narrow QRS duration, which might have been the cause of the low response to CRT. We applied CRT to selected patients with primary cardiomyopathy who had LBBB and a wide QRS duration. They also had classical patterns of mechanical dyssynchrony on echocardiography, such as early septal contraction with lateral wall stretching followed by delayed lateral wall contraction, septal flash, and apical rocking motion.
12) These patients responded well to the CRT; however, their chamber enlargement and dysfunction did not normalize despite early CRT application during infancy. Cardiomyopathy with LBBB might result not only from electrical and mechanical dyssynchrony but also from diseased myocardium.
In contrast, only 56% (5/9) of CHD patients responded to CRT. Previous studies have demonstrated that patients with LV-type systemic ventricles showed a good response to CRT.
13) However, this study showed that systemic LV did not show better response rates than a group of patients with systemic RV or functional single ventricle (83% vs. 50%, p=0.245). Several studies have also reported that systemic RV or univentricular heart is predictive of a poor response to CRT.
9)14) One of the reasons for this may be the higher rates of AV valve regurgitation, resulting in less successful reverse cardiac remodeling.
15) In this study, all 3 patients with a single ventricle had severe AV valve regurgitation with ventricular dysfunction. Among them, 2 patients who underwent CRT and concomitant AV valve replacement showed dramatic improvement in ventricular dysfunction and heart failure, but the other patient who underwent CRT and concomitant AV valve repair showed poor outcomes, resulting in early postoperative death. The latter patient had more than moderate AV valve regurgitation despite repair surgery, which might be the cause of the poor response to CRT and the persistent severe ventricular dysfunction.
Miyazaki et al.
16) showed 88% of good response rate to the CRT with a new ventricular morphology-based strategy for CHD. They grouped patients with CHD with systemic RV, systemic LV, and single ventricular physiology (biventricular, RV + rudimentary LV, LV + rudimentary RV, single RV) into short axis dyssynchrony, long axis dyssynchrony, and interventricular dyssynchrony and decided lead position according to the ventricular morphology and dyssynchrony patterns. It can be a good reference when performing CRT implantation in CHD.
Among the 6 patients with long-term follow-up, there were 3 responders (2 patients with DCM and LBBB, and 1 with CHD), whose shortened QRS duration in the early outcome, prolonged in later follow-up again, even considering that QRS duration normally increased with age, while they remained the responders in the late outcome (
Figure 2). It may reflect the disease progression of underlying cardiomyopathy or conduction delay in areas that do not act as the main pumping part, such as the right ventricular outflow tract. This suggests that early CRT responders deteriorate in further long-term follow-up. Therefore, it is necessary to closely observe their state for a longer period.
The main limitations of this study are its retrospective design and the small number of patients. This resulted in marked heterogeneity of subjects with various underlying heart diseases, age, and the presence of a previous pacemaker, which limits the comparison between subgroups. These are inevitable and common limitations of pediatric CRT studies. Moreover, the measurement of ventricular function by echocardiography requires advanced skills for patients with CHD and has no unified or reliable measurement method, especially in the group with single ventricles and systemic right ventricular anatomy. Despite these limitations, this study had a longer follow-up period to assess the outcomes of CRT in pediatric patients and patients with CHD.
Overall, CRT was associated with improvement in heart function and functional status in pediatric patients and some patients with CHD. In this study, the CRT response rate was significantly high in patients with CRT upgrade in RV-paced complete AV block and DCM with LBBB. However, these results need to be carefully interpreted due to the small sample size, including heterogeneous group. Further larger and longer-term studies are required to demonstrate the favorable factors and predictors of CRT response in pediatric and CHD patients in the future.











 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download