INTRODUCTION
Correct inhaler use is important in the pharmacological treatment of chronic obstructive pulmonary disease (COPD). However, inhaler handling errors and suboptimal adherence are common, and are associated with adverse clinical outcomes such as acute exacerbations.
12 Low inhaler satisfaction is an important risk factor for inhaler handling errors and suboptimal adherence in COPD patients.
34 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines also recommend that patient preference be considered when choosing an inhaler device.
5
Several studies have assessed inhaler satisfaction in chronic airway disease patients. Most of the studies were conducted in asthma patients. High inhaler satisfaction in asthma was associated with good patient compliance as well as better clinical outcomes.
678 However, few studies have assessed factors associated with high inhaler satisfaction among COPD patients.
4910 Although the characteristics of asthma and COPD patient groups may be different, two studies analyzed asthma and COPD patients together.
49 In addition, there are studies that analyzed only dry powder inhalers (DPIs),
9 or received sponsorship by a pharmaceutical company.
10
In this real-world study, we evaluated inhaler satisfaction (all devices available in the Korean market at the start of the study) and determinants of high satisfaction in Korean COPD patients.
DISCUSSION
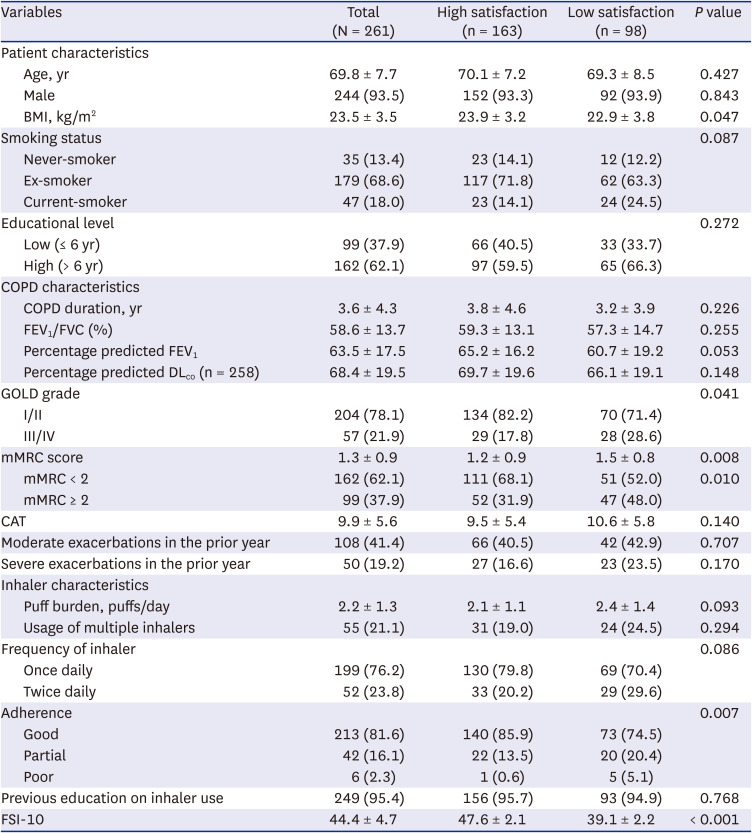
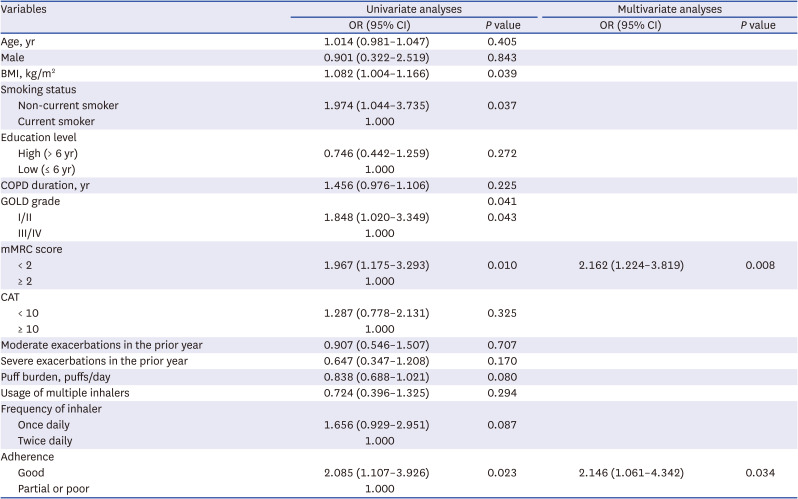
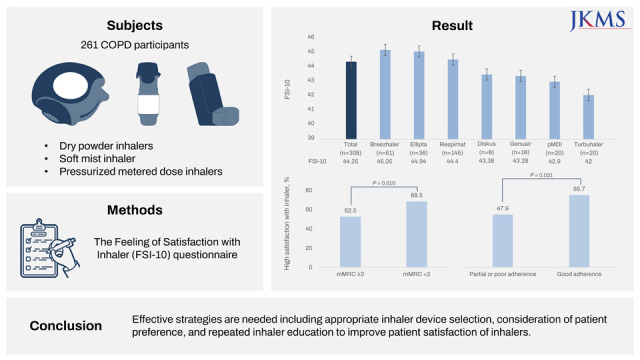
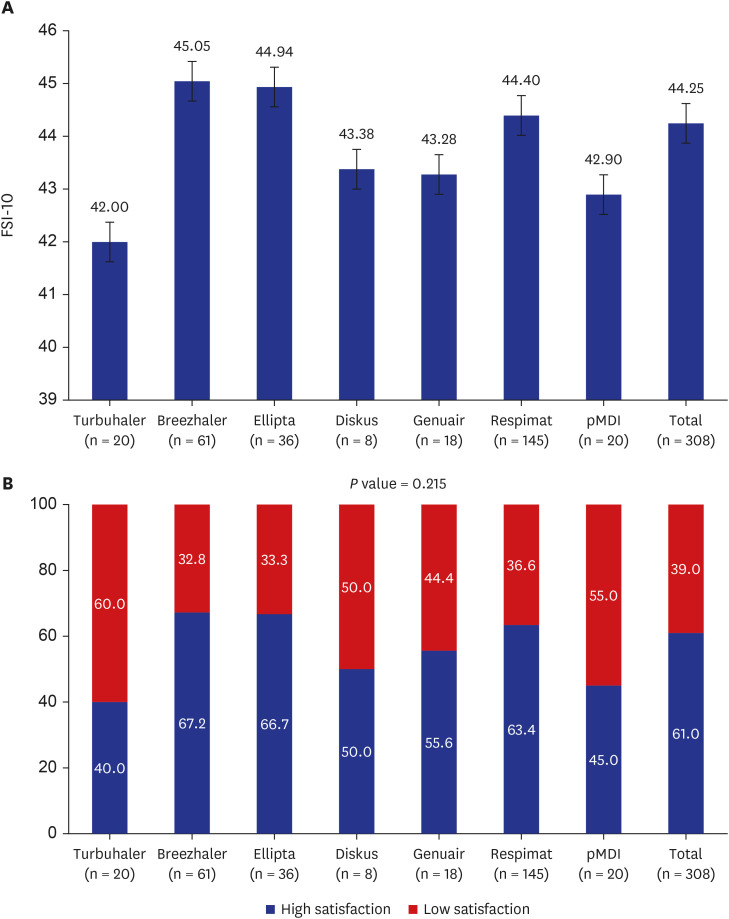
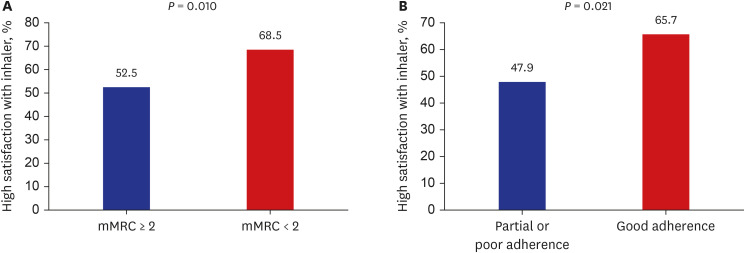
Among the 261 COPD patients, 163 (62.5%) were highly satisfied with their inhaler device. There were no significant differences in FSI-10 scores among the inhaler devices. The percentages of high inhaler satisfaction for the Turbuhaler, Breezhaler, Ellipta, Diskus, Genuair, Respimat, and pMDI were 40.0%, 67.2%, 66.7%, 50.0%, 55.6%, 63.4%, and 45.0%, respectively. Factors independently associated with high inhaler satisfaction included mMRC score < 2 and good inhaler adherence.
The GOLD guidelines for COPD recommend consideration of patient preference when choosing an inhaler device. However, few studies have assessed inhaler satisfaction in COPD patients.
4910
Chrystyn et al.
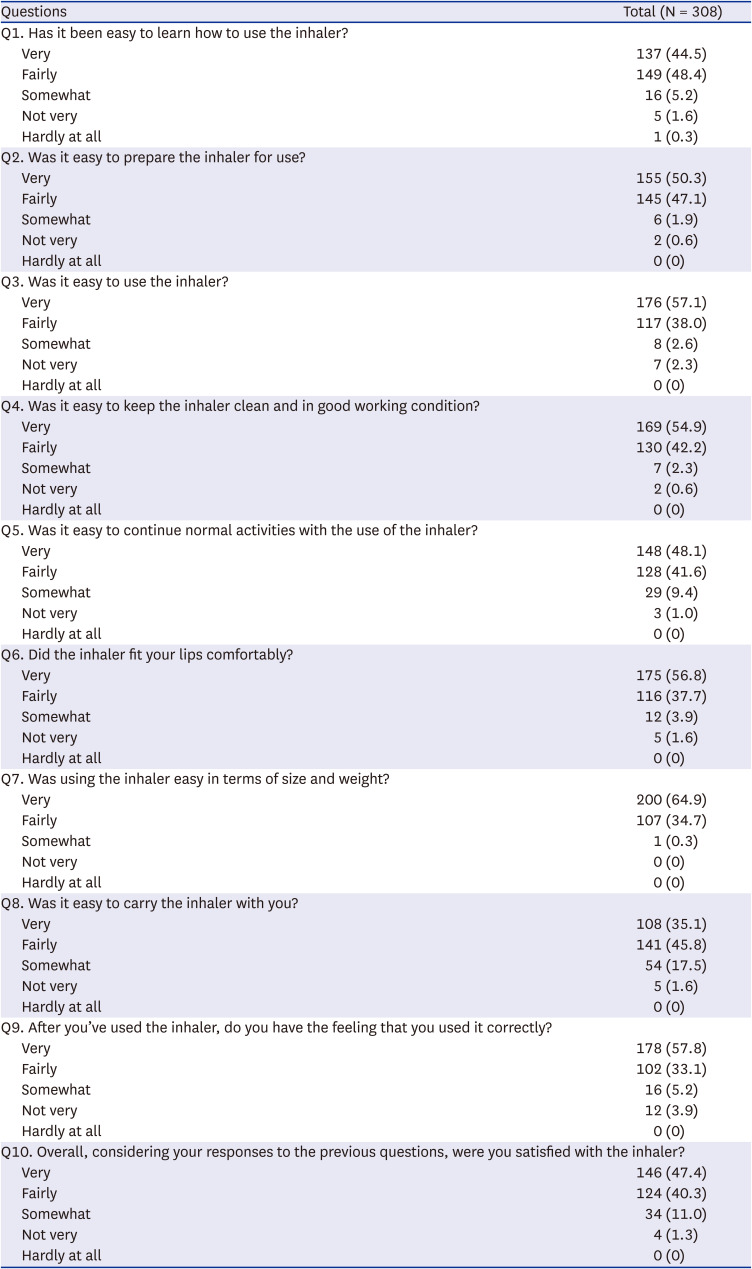
10 examined the relationship between inhaler satisfaction and treatment compliance in 1,443 COPD patients. They used a questionnaire consisting of 13 specific inhaler attributes scored using a 7-point Likert scale, where a score of 1 indicated “not at all satisfied,” and a score of 7 indicated “very satisfied.” In total, 75% of the patients had an overall satisfaction score of at least 5. In addition, there was a significant association between inhaler satisfaction and treatment compliance. Our results were similar; 87.7% of our patients were “very” or “fairly” satisfied, and high inhaler satisfaction was associated with good inhaler adherence.
Zervas et al.
9 estimated satisfaction with different DPI devices in Greek COPD patients using the FSI-10. The total mean FSI-10 was 40.8 ± 6.9 for the Diskus, 44.7 ± 4.4 for the Elpenhaler, and 41.5 ± 5.8 for the Turbuhaler. Severe COPD patients tended to feel greater satisfaction with their inhalers compared to those with mild or moderate disease, irrespective of the device used. In our study, GOLD grade I and II was not associated with high satisfaction, but low dyspnea symptom was associated with high satisfaction. The relationship between severity of COPD and inhaler satisfaction was uncertain until now. Further prospective and longitudinal studies are needed to identify this relationship.
Plaza et al.
4 assessed inhaler satisfaction in 406 asthma and 410 COPD patients using the FSI-10 questionnaire. The asthma group was significantly more satisfied overall with their inhaler (44.1 ± 6.5 vs. 42.0 ± 7.7,
P < 0.001), and significantly more satisfied on 7 of the 10 FSI-10 items. Treatment adherence was correlated with inhaler satisfaction, and a low CAT score in the COPD group was negatively correlated with inhaler satisfaction, suggesting that low CAT was associated with high inhaler satisfaction. Younger age, good disease control, previous inhaler training, and good adherence were associated with high satisfaction in multivariate analyses. In our study, age and previous inhaler training were not associated with inhaler satisfaction. Due to the nature of the baseline characteristics in our study, most participants were elderly COPD patients, the majority of whom (95.4%) had previously received inhaler training; this would have influenced our findings. In addition, it is thought that the large proportions of patients (95.4%) who received inhaler education compared to studies by Plaza et al.
4 (69.5%) is related to the high inhaler satisfaction in our study.
Based on previous studies and our results, increased inhaler satisfaction led to increased inhaler adherence and better clinical outcomes. Organized and repeated inhaler education improved inhaler technique, adherence, and satisfaction.
15 Thus, the importance of education in the use of inhalers cannot be overemphasized.
Multiple inhaler devices are now available, and there may be differences in patient satisfaction among them. A single-center randomized trial compared satisfaction among three DPIs (Genuair, Ellipta, and Breezhaler) in 133 healthy Hong Kong Chinese subjects aged > 40 years. In that study, Breezhaler was rated as more comfortable to use and carry. The overall satisfaction score was higher for Genuair than Ellipta or Breezhaler, where for Genuair clear guidelines on dose and inhalation are provided. However, that study excluded COPD and asthma patients, and validation studies are needed of affected patients.
16 In a Greek study, FSI-10 scores for three DPIs (Diskus, Elpenhaler, and Turbuhaler) were estimated in 561 COPD patients. All three DPIs showed satisfactory results; the Elpenhaler received the highest scores in all age groups, followed by the Turbuhaler and Diskus devices.
9 In our study, the Breezhaler was rated as the best device, followed by the Ellipta and Respimat inhalers, but there were no significant differences among the devices in the rates of high satisfaction. However, as there were large differences in frequency of use among the inhaler devices (Turbuhaler, n = 20; Breezhaler, n = 61; Ellipta, n = 36; Diskus, n = 8; Genuair, n = 18; Respimat, n = 145; and pMDI, n = 20), so the comparative results cannot be considered definitive.
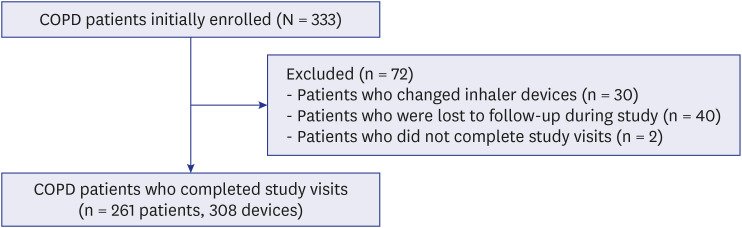
There were several limitations to this study. First, it was a single-center study of COPD patients, and there were differences in the numbers of inhaler devices used among patients. Thus, the results cannot be generalized. In addition, the duration of inhaler device use, which might influence inhaler satisfaction, was not evaluated. Second, of the 333 patients initially enrolled, seventy-two patients were excluded from analysis, which can lead to selection bias. Most of the excluded patients were lost during the study or changed the inhaler. Therefore, the high percentage of good adherence and satisfaction of inhaler in this study may have been overestimated. Third, adherence was self-reported with a single questionnaire according to whether the entire daily dose was taken. This can also cause high percentage of good adherence (81.6%). Previous study also reported extensive discrepancies between self-report and clinician report vs. electronic monitoring in using inhaler treatment.
17 However, our study is meaningful, in that it focused on satisfaction with inhalers only in COPD patients that are widely used in current clinical practice. In addition, unlike other inhaler studies, it has the advantage of analyzing not only DPI but also a significant number of patients using SMI.
In conclusion, inhaler adherence and dyspnea symptom are associated with inhaler satisfaction in COPD patients. Effective strategies are needed including appropriate inhaler device selection, consideration of patient preference, and repeated inhaler education, to improve patient satisfaction of inhalers.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download