Abstract
Traumatic brain injury (TBI) is a critical cause of disability and death worldwide. Many studies have been conducted aimed at achieving favorable neurologic outcomes by reducing secondary brain injury in TBI patients. However, ground-breaking outcomes are still insufficient so far. Because mild-to-moderate hypothermia (32°C–35°C) has been confirmed to help neurological recovery for recovered patients after circulatory arrest, it has been recognized as a major neuroprotective treatment plan for TBI patients. Thereafter, many clinical studies about the effect of therapeutic hypothermia (TH) on severe TBI have been conducted. However, efficacy and safety have not been demonstrated in many large-scale randomized controlled studies. Rather, some studies have demonstrated an increase in mortality rate due to complications such as pneumonia, so it is not highly recommended for severe TBI patients. Recently, some studies have shown results suggesting TH may help reperfusion/ischemic injury prevention after surgery in the case of mass lesions, such as acute subdural hematoma, and it has also been shown to be effective in intracranial pressure control. In conclusion, TH is still at the center of neuroprotective therapeutic studies regarding TBI. If proper measures can be taken to mitigate the many adverse events that may occur during the course of treatment, more positive efficacy can be confirmed. In this review, we look into adverse events that may occur during the process of the induction, maintenance, and rewarming of targeted temperature management and consider ways to prevent and address them.
In adult out-of-hospital cardiac arrest survivors with ventricular tachycardia or fibrillation, when TH (32°C–34°C for 12–24 hours) is applied after resuscitation, it is well-known to improve neurologic outcomes by reducing reperfusion injury (hypoxic-ischemic cascade), and it is therefore recommended. Based on this situation, many studies have been conducted to confirm the effects of therapeutic hypothermia (TH) or targeted temperature management (TTM) on severe traumatic brain injury (TBI) treatment.
The efficacy of TH has not been demonstrated so far to improve neurological outcomes in patients with TBI in multicenter randomized controlled study (RCT) [1-4]. For this reason, early (within 2.5 hours), short-term (48 hours post-injury), and prophylactic hypothermia are not recommended to improve outcomes in patients with diffuse injury in the Guidelines for the Management of Severe Traumatic Brain Injury [2,5]. Nonetheless, many institutions are still using TTM for the purpose of neuroprotection, intracranial pressure (ICP) control, and decreasing fever burden in patients with severe TBI.
ICP control and reducing fever burden are very important to prevent secondary brain injury in patients with severe TBI. Fever is commonly found in neurological ICU patients, increases the risk of complications, and is usually associated with unfavorable clinical outcomes [6-8]. Recently, the ability to stably control patients’ body temperatures has become possible thanks to the development of equipment. TTM is considered to be able to help improve clinical outcomes by reducing fever burden. In this review, we would like to examine the factors that should be considered in the process of applying TH or TTM in TBI patients.
The exact reason why TH in TBI does not work is unknown. Some researchers are seeking answers in the mechanisms of TBI. To date, the disease in which TH is known to improve neurologic outcomes is out-of-hospital cardiac arrest [9,10]. For this reason, post-cardiac arrest care guidelines were modified to recommend target temperatures of 32°C to 36°C [11-13]. However, compared to patients with these diseases and TBI, the pathological environment of the brain is different, so the effect does not seem to be perfect.
In the case of cardiac arrest, abnormalities in brain cell function occur due to transient global ischemia; therefore, there is little structural destruction in brain cells. When the cardiac arrest time is short, TH after successful resuscitation improves clinical outcomes by preventing secondary brain injury by blocking the hypoxic-ischemic cascade. However, in the case of TBI, TH effects can be limited because there are several lesions, such as permanent structural destruction, cerebral hemorrhage, ischemic lesions, delayed edema, etc., rather than single ischemia. And there seems to be a limitation in recovery since the primary injury caused by the initial insult is severe (Table 1) [14].
Brain edema and secondary brain injury in TBI patients persist for hours to days. Therefore, when TH or TTH is applied for the purpose of these cases, it is recommended to be maintained for a long time rather than post-resuscitation [2,11]. In RCTs on prolonged mild hypothermia and slow rewarming in patients with severe TBI, tight hemodynamic management and slow rewarming, together with prolonged TH (32°C–34°C), did not improve the neurological outcomes or reduce mortality compared with strict temperature control (35.5°C–37°C) [1,2]. Although there are no clear guidelines on when to start and how long to maintain decreased ICP by controlling brain edema to help neurological recovery and reduce the length of stay in the ICU, maintaining TTM within normal body temperature (36°C–37.5°C) can be considered to reduce fever burden and variability from the beginning. When there’s no response to other existing medical treatments, it is recommended to perform induced hypothermia at 32°C–35°C to control brain edema and ICP [5,15]. In this case, the treatment should be maintained for days, and it would be good to do a slow rewarming with ICP monitoring [15,16]. Hypothermia combined with craniotomy may improve neurologic outcomes in patients with hematomas and severe TBI [3,4]. Overall, cooling before decompression surgery weakens some of the reduction of reperfusion injury.
TH can be divided into three phases: phase 1 (induction), phase 2 (maintenance), and phase 3 (rewarming) [14,17]. Let’s take a look at what to consider at each stage [14,18].
If it is decided to do TH in refractory increased ICP, the target temperature should be reached by maximizing the speed of cooling as much as possible [10]. Although there are many methods for this, servo-regulatory non-invasive surface cooling devices and invasive endovascular cooling devices are mainly used. In addition, the application of ice bags and local cooling methods exist, but they are not commonly used. In the case of invasive endovascular cooling devices, there is a risk of catheter-related thrombosis and infection, and in the case of surface cooling devices, there is a risk of skin necrosis and soreness caused by peripheral vasoconstriction of the skin [19].
In a study, a large ice-cold fluid infusion (4°C, 20–30 ml/kg) did not increase the incidence of pulmonary edema but increased the incidence of rearrests [10,19]. Some studies insist that rapid induction decreases the risks and consequences of short-term side effects, such as shivering and metabolic disorders [19]. But in many cases, it is necessary to consider edema and cardiovascular effects due to volume-overload in patients with severe TBI.
TH should be performed based on the core temperature. The optimal temperature measurement site should accurately reflect the core temperature, be non-invasive, and be measured continuously. The most commonly used sites are the esophagus, bladder, and rectum, in that order [9,20]. Direct measuring equipment using sensors attached to the scalp has recently been used for brain temperature [21].
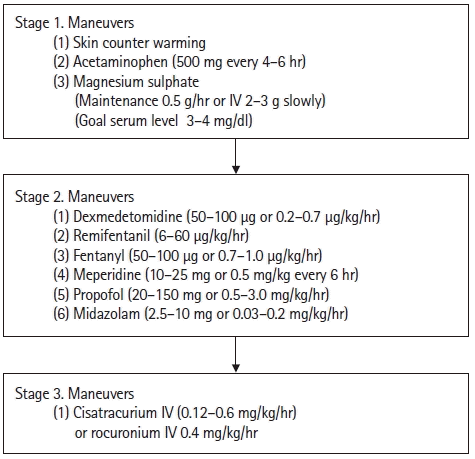
Because shivers can increase the rate of metabolism, oxygen consumption, excess breathing, heart rate, and a general stress-like response, shivering management, such as sedatives, anesthetics, opiates, magnesium, muscle paralyzers, and various other drugs, are important during TH [19,22]. A shivering assessment scale is provided in Table 2 [23]. Drugs and skin counter warming for shivering mitigation are organized in Figure 1. However, many patients with severe TBI are under sedation therapy, so it is sometimes difficult to identify them.
After reaching the target temperature after induction, it is important to reduce body temperature variation and shivering before rewarming. Also, it is very important to prevent and treat various complications that may occur during TH. The most used surface cooling device, gel pads attached to the skin, is non-invasive and convenient but has disadvantages in that skin necrosis and shivering can occur more easily. Endovascular devices using endovascular insertion and a catheter are invasive and can cause complications, such as infection, bleeding, and venous thrombosis, therefore, it should be used with caution. To decrease temperature variability, using temperature-modulating devices with servo-controls and gradient temperature changes is recommended [15]. During this phase, potential complications caused by hypothermia can occur (Table 3). Let’s study more about them.
In the early stage of TH, blood pressure rises due to peripheral vasoconstriction and hypotension thereafter due to hypovolemia caused by cold diuresis decreases cardiac output and induces arrhythmia including bradycardia [20,24]. If hypovolemia is corrected, mild to moderate hypothermia does not decrease myocardial contractility or induce hypotension [25]. Therefore, it is an important point to correct hypovolemia. Osmotic agents are often used for patients with severe TBI and central diabetes insipidus (DI) due to ICP, so it is important to check volume status and properly manage volume treatment. According to a recent publication, hypertonic saline enhances ICP and cerebral perfusion pressure more than mannitol does, so, because of the effect of volume expansion, hypertonic saline can be useful during TH [26].
Many patients with severe TBI have the risk of catheter-related infection related to multiple trauma wounds, postoperative status, central line, intubation tube, foley catheter, etc. Pneumonia is the most common complication due to aspiration in the unconscious state of the early stage of severe TBI. In these conditions, TH increases the risk of infection due to immunosuppression and leukopenia. Therefore, to check whether infection is progressing during TH maintenance, protocolized care for infection monitoring should be needed. If infection control is not proper and goes to sepsis, the mortality risk increases significantly.
Existing tests to monitor infection progression include white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), blood-culture, etc. However, it is difficult to determine the progression of infection with these tests because fever pattern and WBC count can be influenced by TH. In addition, ESR/CRP can rise non-specifically in the case of multiple trauma, and the results of blood culture are slow, making treatment with proper timing difficult. As such, existing blood tests related to inflammation have limitations, so additional research on new methods is needed. One sign of progressing infection can be a sudden increase in the workload of the cooling device, such as a decrease in water temperature and an increase in noise levels of the device. If an infection is developing, our body attempts to generate fever and cause shivering [25]. There is a report that the use of prophylactic antibiotics decreased the incidence of pneumonia 4-fold [28]. But, to date, the best timing of antibiotic therapy and the drug choice are still unclear, and prophylactic use of broad-spectrum antibiotics is thought to be necessary for patients with severe TBI.
TH increases bleeding risk by inducing thrombocytopenia, platelet dysfunction, and mild coagulopathy [25,29]. Patients with severe TBI often have consumptive coagulopathy due to multiple trauma, and many of them also have traumatic intracerebral hemorrhage or delayed hemorrhage. It should be considered that TH during the early stage of trauma may adversely affect patients because of thrombocytopenia and mild coagulopathy, increased bleeding. For this, personalized transfusion can be helpful for proper coagulation in the early stage of trauma. Also, thromboelastometry can be one way to do this [30].
TH causes many electrolyte changes and hyperglycemia. Decreased K+, P+, and Mg2+ and increased glucose are most common [31,32]. In particular, severe electrolyte depletion, which is partly due to an increase in the amount of discharge caused by cold diuresis, should be thoroughly corrected because it causes cardiac arrhythmia and is life-threatening. So, electrolyte levels should be monitored frequently, and prophylactic electrolyte supplementation should be considered. It is recommended to maintain 4.0 mEq/L or more before TH induction and 3.0–3.5 mEq/L during maintenance [15]. The reason why K+ should be maintained in the low limit of the normal range is that decreased potassium by intracellular shifting during rewarming can cause rebound hypokalemia. Besides, tests for Mg and P should be properly performed and maintained during TH [32]. In the case of refractory K+ replacement, it must be confirmed that hypomagnesemia is not the cause. Because magnesium is necessary for sodium, potassium, and calcium to move into and out of cells, hypomagnesemia results in hypocalcemia and hypokalemia.
Also, TH can increase insulin resistance and cause hyperglycemia. Severe TBI can be accompanied by stress-induced hyperglycemia in the early stage, so it is necessary to control hyperglycemia, minimizing glycemic variability. Appropriate glycemic control methods and insulin types have not yet been determined, but it is better to measure glucose levels more frequently, and continuous monitoring can be considered [31].
Cerebral metabolism decreases by 6% to 10% for each 1°C reduction in body temperature during cooling [33]. Accordingly, the requirements for oxygen and glucose are decreased. If the ventilator setting is not properly adjusted, accidental hyperventilation and hyperglycemia can be maintained due to decreased oxygen and glucose consumption during TH, and it induces secondary brain injury by vasoconstriction.
Another point of consideration is the oxygen dissociation curve of hemoglobin. In a hypothermic state, the solubility of oxygen and carbon dioxide increases. However, since arterial blood test results are tested at 37℃, pH and carbon dioxide levels are different from the actual values in the hypothermic state [34]. PaO2 is decreased by 5 mm Hg for each degree below 37°C, and PaCO2 is decreased by 2 mm Hg for each degree below 37°C. Alpha-stat means temperature-uncorrected arterial pCO2, and pH-stat means temperature-corrected arterial pCO2 [25]. Strictly applied alpha-stat management can lead to hyperventilation and cerebral vasoconstriction; strict pH-stat management can lead to hypercapnia, cerebral vasodilation, and increased ICP. Which option is preferable may depend on the precise nature of the neurological injury and the presence or absence of brain edema. Excessive hypocapnia can increase ischemia in injured areas of the brain, while excessive hypercapnia can increase brain edema; thus, both can cause additional brain injury [25]. An example situation was shown in Figure 2.
Although there’s controversy as to whether alpha-stat or pH-stat are superior, the recent guidelines recommend pH-stat [12,15]. Arterial blood gas measurements should be temperature-corrected during TH; also, increased PH makes the oxygen dissociation curve shift to the left. In this case, the oxygen affinity of hemoglobin increases, and oxygen delivery to the tissues decreases. Therefore, it is necessary to maintain oxygen concentration at the upper limit in the normal range.
TH could decrease the function of physiologic enzymes and affect changes in drug clearance. Because various drugs including anticonvulsants, antibiotics, analgesics, and sedatives are used in patients with severe TBI, their therapeutic efficacy and serum concentrations should be monitored. In addition, it should be considered that the effect of these drugs may last longer than expected [30].
Nonconvulsive status epilepticus (i.e., epileptic activity without obvious clinical signs and symptoms) frequently occurs in patients with TBI [35]. Hypothermia acts as neuroprotection, suppresses epileptic activity, and spreads nervous system depression-like depolarization suppression [25,36]. Therefore, the use of prophylactic anticonvulsants is not recommended. Vasoconstriction of the skin caused by hypothermia could affect the occurrence of wound infection and bed sores [25]. To prevent this, warming of the exposed skin could be an option to try in the case of using invasive endovascular cooling devices or surface-cooling machine (Arctic Sun; Medivance Inc., Louisville, CO, USA). Bowel function and delayed gastric emptying caused by TBI may occur during hypothermia. However, a proper treatment strategy for this area has not yet been suggested.
Considerations during rewarming are opposed to induction. To reduce side effects, rewarming should be performed slowly. In general, rewarming is performed at 0.25°C per hour, but if there’s a concern about ICP, it may be maintained more slowly at <0.1°C per hour [25]. Slow rewarming for over 48 hours might improve the neurological outcomes of prolonged TTM in patients with TBI and an evacuated hematoma. Further studies are needed to determine the optimal rewarming protocol in patients with TBI [37]. If all K+-containing fluids are needed to prevent or stop hyperkalemia, the management of hypotension due to vasodilation, hypoglycemia, arrhythmias, shivering, or seizure should be prepared for. It is recommended to maintain 24–48 hours of normothermia after rewarming and then apply titration of analgesics and sedatives to prevent rebound fever [20].
So far, TH has not yet been proven clinically effective in improving neurologic outcomes. However, appropriate thermoregulation with TTM (TH and intensive normothermia) might have a critical role in the treatment of patients with severe TBI through ICP control and neuroprotection by various mechanisms. The medical team in charge of neurointensive care should understand the physiologic response to hypothermia and provide appropriate supportive care according to well-organized protocols to reduce adverse effects.
▪ Targeted temperature management is one of the most promising neuroprotective methods to prevent secondary brain injury in patients with traumatic brain injury.
▪ Although many studies have not proven its effectiveness clearly so far, and there have been many problems due to side effects, its complications and side effects have been significantly reduced as general supportive technology develops.
▪ It is expected that clinical studies including patients with the proper indications and well-organized protocols for targeted temperature management can be conducted.
REFERENCES
1. Maekawa T, Yamashita S, Nagao S, Hayashi N, Ohashi Y, Brain-Hypothermia Study Group. Prolonged mild therapeutic hypothermia versus fever control with tight hemodynamic monitoring and slow rewarming in patients with severe traumatic brain injury: a randomized controlled trial. J Neurotrauma. 2015; 32:422–9.

2. Cooper DJ, Nichol AD, Bailey M, Bernard S, Cameron PA, Pili-Floury S, et al. Effect of early sustained prophylactic hypothermia on neurologic outcomes among patients with severe traumatic brain injury: the POLAR Randomized Clinical Trial. JAMA. 2018; 320:2211–20.

3. O’Leary R, Hutchinson PJ, Menon D. Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med. 2016; 374:1383–4.

4. Clifton GL, Coffey CS, Fourwinds S, Zygun D, Valadka A, Smith KR Jr, et al. Early induction of hypothermia for evacuated intracranial hematomas: a post hoc analysis of two clinical trials. J Neurosurg. 2012; 117:714–20.

5. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017; 80:6–15.

7. Diringer MN, Reaven NL, Funk SE, Uman GC. Elevated body temperature independently contributes to increased length of stay in neurologic intensive care unit patients. Crit Care Med. 2004; 32:1489–95.

8. Kilpatrick MM, Lowry DW, Firlik AD, Yonas H, Marion DW. Hyperthermia in the neurosurgical intensive care unit. Neurosurgery. 2000; 47:850–6.

9. Lascarrou JB, Merdji H, Le Gouge A, Colin G, Grillet G, Girardie P, et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N Engl J Med. 2019; 381:2327–37.

10. Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002; 346:557–63.

11. Berg KM, Soar J, Andersen LW, Böttiger BW, Cacciola S, Callaway CW, et al. Adult advanced life support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation. 2020; 142(16_suppl_1):S92–139.
12. Nolan JP, Sandroni C, Böttiger BW, Cariou A, Cronberg T, Friberg H, et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med. 2021; 47:369–421.

13. Panchal AR, Bartos JA, Cabañas JG, Donnino MW, Drennan IR, Hirsch KG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020; 142(16_suppl_2):S366–468.
14. Choi HA, Badjatia N, Mayer SA. Hypothermia for acute brain injury: mechanisms and practical aspects. Nat Rev Neurol. 2012; 8:214–22.

15. Madden LK, Hill M, May TL, Human T, Guanci MM, Jacobi J, et al. The implementation of targeted temperature management: an evidence-based guideline from the Neurocritical Care Society. Neurocrit Care. 2017; 27:468–87.

16. Staykov D, Wagner I, Volbers B, Doerfler A, Schwab S, Kollmar R. Mild prolonged hypothermia for large intracerebral hemorrhage. Neurocrit Care. 2013; 18:178–83.

19. Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009; 37:1101–20.

20. Cariou A, Payen JF, Asehnoune K, Audibert G, Botte A, Brissaud O, et al. Targeted temperature management in the ICU: guidelines from a French expert panel. Ann Intensive Care. 2017; 7:70.

21. Schell-Chaple HM, Liu KD, Matthay MA, Puntillo KA. Rectal and bladder temperatures vs forehead core temperatures measured with SpotOn monitoring system. Am J Crit Care. 2018; 27:43–50.

22. De Witte J, Sessler DI. Perioperative shivering: physiology and pharmacology. Anesthesiology. 2002; 96:467–84.
23. Badjatia N, Strongilis E, Gordon E, Prescutti M, Fernandez L, Fernandez A, et al. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke. 2008; 39:3242–7.

24. Rittenberger JC, Polderman KH, Smith WS, Weingart SD. Emergency neurological life support: resuscitation following cardiac arrest. Neurocrit Care. 2012; 17 Suppl 1:S21–8.

25. Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009; 37(7 Suppl):S186–202.

26. Mangat HS, Wu X, Gerber LM, Schwarz JT, Fakhar M, Murthy SB, et al. Hypertonic saline is superior to mannitol for the combined effect on intracranial pressure and cerebral perfusion pressure burdens in patients with severe traumatic brain injury. Neurosurgery. 2020; 86:221–30.

27. Kuroda Y, Kawakita K. Targeted temperature management for postcardiac arrest syndrome. J Neurocrit Care. 2020; 13:1–18.

28. Gagnon DJ, Nielsen N, Fraser GL, Riker RR, Dziodzio J, Sunde K, et al. Prophylactic antibiotics are associated with a lower incidence of pneumonia in cardiac arrest survivors treated with targeted temperature management. Resuscitation. 2015; 92:154–9.

29. Rohrer MJ, Natale AM. Effect of hypothermia on the coagulation cascade. Crit Care Med. 1992; 20:1402–5.

30. Crochemore T, Piza FM, Rodrigues RD, Guerra JC, Ferraz LJ, Corrêa TD. A new era of thromboelastometry. Einstein (Sao Paulo). 2017; 15:380–5.

31. Bruining HA, Boelhouwer RU. Acute transient hypokalemia and body temperature. Lancet. 1982; 2:1283–4.
32. Polderman KH, Peerdeman SM, Girbes AR. Hypophosphatemia and hypomagnesemia induced by cooling in patients with severe head injury. J Neurosurg. 2001; 94:697–705.

33. Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab. 2003; 23:513–30.

34. van der Worp HB, Macleod MR, Kollmar R, European Stroke Research Network for Hypothermia (EuroHYP). Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials? J Cereb Blood Flow Metab. 2010; 30:1079–93.

35. Vespa PM, Nuwer MR, Nenov V, Ronne-Engstrom E, Hovda DA, Bergsneider M, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999; 91:750–60.

Figure 2.
Different management plans according to alpha-stat and pH-stat. ICP: intracranial pressure.
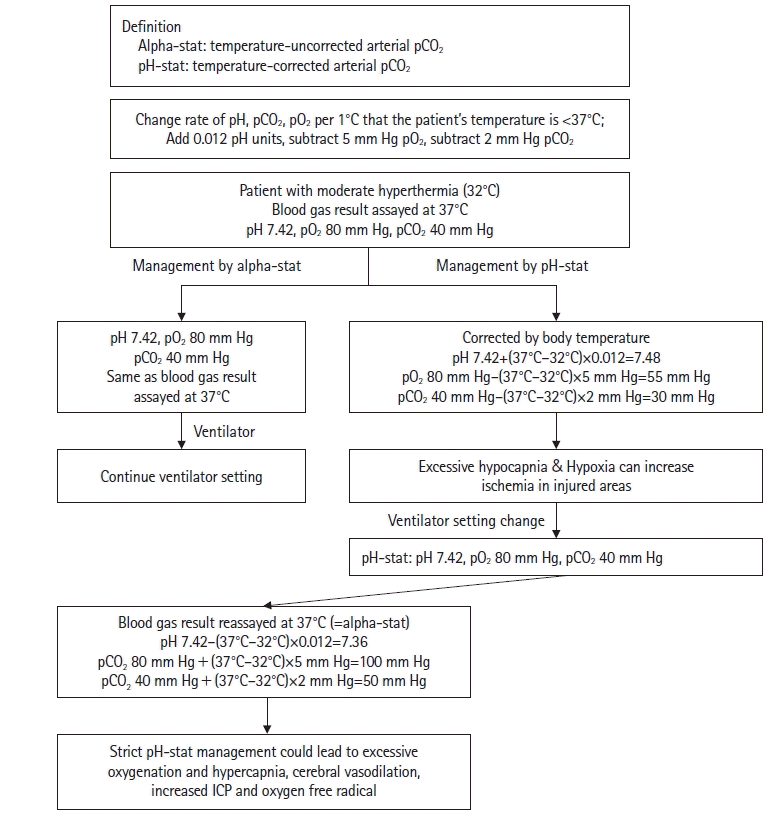
Table 1.
Difference between the two conditions
Table 2.
Bedside Shivering Assessment Scale [23]
Table 3.
Summary of potential complications of IH




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download