Abstract
Background
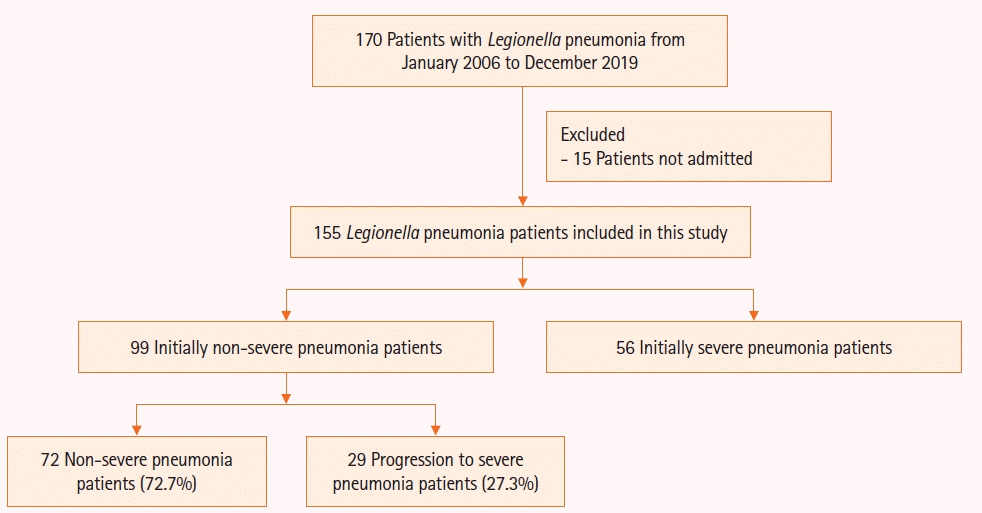
Methods
Results
REFERENCES
Table 1.
| Characteristics | Value |
|---|---|
| Number of patients | 155 |
| Age (yr) | 64.9±12.9 |
| Male | 118 (76.1) |
| Body mass index (kg/m2) | 22.2±3.4 |
| Current smoker | 19 (12.3) |
| Category of pneumonia | |
| Community-acquired pneumonia | 70 (45.2) |
| Healthcare-associated pneumonia | 54 (34.8) |
| Hospital-acquired pneumonia | 31 (20.0) |
| Comorbidity | |
| Immunocompromised statea) | 89 (57.4) |
| Diabetes mellitus | 39 (25.2) |
| Chronic lung disease | 30 (19.4) |
| Chronic obstructive pulmonary disease | 12 (40.0) |
| Interstitial lung disease | 8 (26.7) |
| Bronchiectasis | 5 (16.7) |
| Emphysema | 3 (10.0) |
| Chronic bronchitis | 1 (3.3) |
| Asthma | 1 (3.3) |
| Chronic kidney disease | 26 (16.8) |
| Chronic heart disease | 18 (11.6) |
| Liver cirrhosis | 13 (8.4) |
| Clinical finding | |
| Fever | 104 (67.1) |
| Sputum | 99 (63.9) |
| Cough | 97 (62.6) |
| Dyspnea | 80 (51.6) |
| Altered mental status | 22 (14.2) |
| Chest discomfort | 17 (11.0) |
| Diarrhea | 17 (11.0) |
| Headache | 5 (6.9) |
| Laboratory finding | |
| Leukocytes (/mm3) | 9,200 (4,100–13,500) |
| Platelets (×103/mm3) | 174.0 (91.5–238.5) |
| Blood urea nitrogen (mg/dl) | 24.0 (17.5–37.5) |
| Sodium (mmol/L) | 134.0 (130.0–137.0) |
| Lactate dehydrogenase (U/L) | 362.0 (255.0–481.0) |
| C-reactive protein (mg/dl) | 16.8 (8.3–27.2) |
| PaO2/FiO2 ratio | 276.2 (206.7–342.8) |
| Radiologic finding | |
| Multilobar involvement | 104 (67.1) |
| Bilateral involvement | 91 (58.7) |
| Pleural effusion | 25 (16.1) |
| Pneumonia severity | |
| CURB-65 score | 2 (1–2) |
Values are presented as mean±standard deviation, number (%), or median (interquartile range).
PaO2: partial pressure of arterial oxygen; FiO2: fraction of inspired oxygen; CURB-65: confusion, uremia, blood pressure, age ≥65 years.
a) An immunocompromised state was defined if one of the following criteria were met: (1) receiving immunosuppressants daily, including corticosteroids; (2) infection with human immunodeficiency virus; (3) receiving solid organ or hematopoietic stem cell transplantation; (4) receiving chemotherapy for underlying malignancy during the previous 6 months; and (5) presence of other underlying immunodeficiency disorders.
Table 2.
| Characteristics | Progressed | Non-progressed | P-value |
|---|---|---|---|
| Number of patients | 28 | 69 | - |
| Age (yr) | 66.0±11.2 | 63.0±14.2 | 0.327 |
| Male | 24 (85.7) | 49 (71.0) | 0.207 |
| Body mass index (kg/m2) | 23.5±3.4 | 22.2±3.4 | 0.098 |
| Current smoker | 2 (7.1) | 10 (14.5) | 0.512 |
| Immunocompromised statea) | 20 (71.4) | 34 (51.5) | 0.119 |
| Chronic lung disease | 5 (17.9) | 15 (21.7) | 0.880 |
| Leukocytes (/mm3) | 7,850 (3,150–10,775) | 9,800 (6,500–15,100) | 0.040 |
| Platelets (×103/mm3) | 102 (44.5–180.5) | 193 (131–269) | <0.001 |
| Protein (mg/dl) | 5.9±0.9 | 6.3±1.0 | 0.361 |
| Albumin (mg/dl) | 2.5±0.6 | 2.8±0.6 | 0.203 |
| Sodium (mmol/L) | 134.5 (131.8–136.2) | 134.0 (130.0–137.0) | 0.984 |
| Blood urea nitrogen (mg/dl) | 20.5 (18.0–29.0) | 22 (22.0–34.0) | 0.837 |
| PaO2/FiO2 ratio <300 | 12 (42.9) | 18 (26.1) | 0.169 |
| Bilateral involvement | 17 (60.7) | 29 (42.0) | 0.148 |
| Pleural effusion | 2 (7.1) | 10 (14.5) | 0.512 |
| Fluoroquinolone use | 24 (85.7) | 60 (87.0) | >0.999 |
| Macrolide use | 10 (35.7) | 24 (34.8) | >0.999 |
| Both fluoroquinolone and macrolide use | 15 (21.7) | 8 (28.6) | 0.650 |
| CURB-65 score | 2 (1–2) | 1 (1–2) | 0.236 |
Values are presented as mean±standard deviation, number (%), or median (interquartile range).
PaO2: partial pressure of arterial oxygen; FiO2: fraction of inspired oxygen; CURB-65: confusion, uremia, blood pressure, age ≥65 years.
a) An immunocompromised state was defined if one of the following criteria were met: (1) receiving immunosuppressants daily, including corticosteroids; (2) infection with human immunodeficiency virus; (3) receiving solid organ or hematopoietic stem cell transplantation; (4) receiving chemotherapy for underlying malignancy during the previous 6 months; and (5) presence of other underlying immunodeficiency disorders.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download