Abstract
Background
Pediatric intensive care units (PICUs), where children with critical illnesses are treated, require considerable manpower and technological infrastructure in order to keep children alive and free from sequelae.
Methods
In this retrospective comparative cohort study, hospital records of patients aged 1 month to 18 years who died in the study PICU between January 2015 and December 2019 were reviewed.
Results
A total of 2,781 critically ill children were admitted to the PICU. The mean±standard deviation age of 254 nonsurvivors was 64.34±69.48 months. The mean PICU length of stay was 17 days (range, 1–205 days), with 40 children dying early (<1 day of PICU admission). The majority of nonsurvivors (83.9%) had comorbid illnesses. Children with early mortality were more likely to have neurological findings (62.5%), hypotension (82.5%), oliguria (47.5%), acidosis (92.5%), coagulopathy (30.0%), and cardiac arrest (45.0%) and less likely to have terminal illnesses (52.5%) and chronic illnesses (75.6%). Children who died early had a higher mean age (81.8 months) and Pediatric Risk of Mortality (PRISM) III score (37). In children who died early, the first three signs during ICU admission were hypoglycemia in 68.5%, neurological symptoms in 43.5%, and acidosis in 78.3%. Sixty-seven patients needed continuous renal replacement therapy, 51 required extracorporeal membrane oxygenation support, and 10 underwent extracorporeal cardiopulmonary resuscitation.
Pediatric intensive care units (PICUs) are units that have the manpower and technological infrastructure to care for critically ill children. The survival rates of patients admitted to the PICU have increased over the years [1]. The reasons for critically ill children to stay in the ICU include the use of inotropes, vasopressors, multiple antiepileptics, respiratory and circulatory monitoring, invasive mechanical ventilation (IMV) and non-invasive mechanical ventilation (NIV), extracorporeal membrane oxygenation (ECMO), continuous renal replacement therapy (CRRT), and plasma exchange (PEX). In addition, patients are followed up via liver, heart, and lung transplantation as well as heart surgery, neurosurgery, and other major surgical procedures. Mortality rates have decreased over the years with all this monitoring, knowledge, and application of advanced treatment methods [2].
Valli et al. [3] reported that the more common cause of death was cardiovascular syndrome (30%) in the early deaths group and sepsis (27%) and acute respiratory failure (27%) in the late deaths group. It is particularly important to uncover the characteristics of early deaths because these patients are often withdrawn from studies, often due to insufficient time for approval and treatment [4]. In this study, we aimed to evaluate the primary diseases, clinical characteristics, and other factors affecting mortality, comparing patients who experienced early and late mortality in the PICU during the study period.
The study was approved by the Ethics Committee of the Ankara University School of Medicine. Consent for study and publication was obtained from the families of participants.
Hospital records of patients aged 1 month to 18 years who died in PICU between January 2015 and December 2019 were reviewed. Information on patients' demographic features, primary diagnoses, and comorbid illnesses was recorded. In addition, we recorded all of the following: the service where the patients came from, the indication for PICU admission, the PICU length of stay (LOS), vital signs on admission to the PICU (arterial pressure, heart rate, and body temperature), hypoglycemia, neurological findings, acidosis, liver dysfunction, coagulation disorder, cardiac arrest, and inotrope requirements; nosocomial infections (NIs) and nutritional status; the findings of laboratory investigations obtained within the first 30 minutes of admission (blood count, biochemistry, coagulation profile, blood gas analysis, and imaging); the durations of (MV and NIV; the use of vasoactive drugs, catheters, and drains; the occurrence of acute kidney injury; the use of CRRT, PEX, and ECMO; the presence of proven infections on admission, duration of antibiotics, and history of previous surgery; and neurological findings, such as changes in consciousness, seizures, and intracranial hemorrhage. The mortality score (Pediatric Risk of Mortality [PRISM] III) and vasoactive-inotropic score (VIS)=dopamine dose (µg/kg/min)+dobutamine dose (µg/kg/min)+100×adrenaline dose (µg/kg/min)+10×milrinone dose (µg/kg/min)+10,000×vasopressin dose (units/kg/min)+100×noradrenaline dose (µg/kg/min) were calculated at 24 hours after first admission to the ICU. A death within the first 24 hours of PICU admission was considered an “early death”; any death after the first day was considered a “late death.”
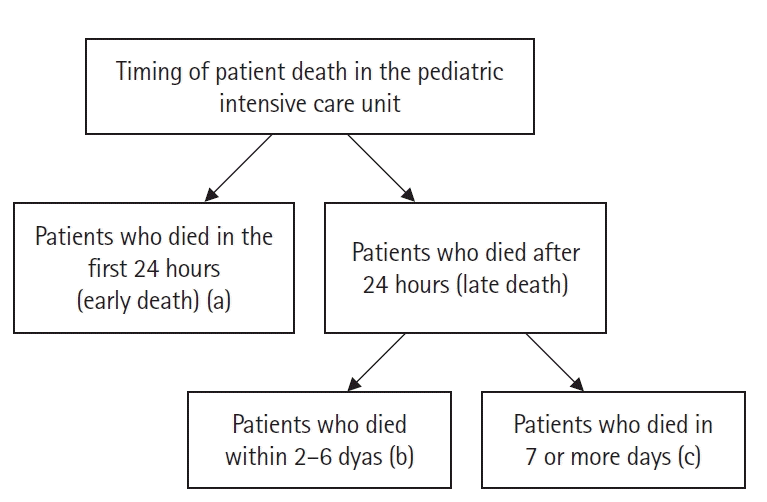
The "timing" of death was recorded as the patient's LOS. The time of death was divided into three categories according to the time of PICU admission as 1 (a), 2–6 (b), or ≥7 (c) days after admission (Figure 1).
The SPSS 11.0 statistics package program (SPSS Inc., Chicago, IL, USA) was used to evaluate the data. Data were checked for normality using the Shapiro-Wilk test. Normally distributed variables were expressed as mean±standard deviation (SD) values, and variables not normally distributed were expressed as median (25th–75th) values. The Mann-Whitney U-test was used for two-point comparison of non-normally distributed data. The P<0.05 was considered to be statistically significant.
There were 254 deaths during the 5-year study period, and the mean mortality rate was 9.13%. Mortality numbers and rates by year are shown in Figure 2. There were 138 boys (54.3%) and 116 girls (45.7%) enrolled in the study with a mean±SD age of 64.34±69.48 months and mean±SD PRISM III score of 24±17.04 points. Most patients (83%) had underlying illnesses, and the remaining 17% were previously healthy. Sepsis (n=12, 4.7%), respiratory disease (n=7, 2.5%), and neurological disease (n=11, 4.3%) were the leading causes of death in previously healthy children, while malignancy (n=59, 23.2%), heart disease (n=64, 25.1%), and neurological disease (n=28, 11.0%) were the leading causes of death in patients with underlying illnesses. Fifty-eight patients (22.8%) were referred from other hospitals through the national emergency transfer system, 55 patients (21.7%) were admitted from the emergency room, 38 patients (14.9%) were admitted after surgery, 11 (4.1%) were transferred from the neonatal ICU, and 94 patients (37.0%) were admitted from pediatric wards (Table 1).
The early death rates of patients admitted from the emergency department (30%) and wards (50%) were higher than those of patients who were referred from other centers (10%), the operating room (10%), and the neonatal ICU (0%) (Table 2, Figure 3A). Forty patients died early (15.7%). Children who died early had a higher median age (81.8 months; range, 2–248 months) and PRISM III score (37 points; range, 8–78 points) (Table 2).
Patients were classified according to the Society of Critical Care Medicine ICU admission prioritization model [5]. According to this, the common indications of hospitalization were respiratory failure (n=77, 30.3%; priority 1), sepsis (n=36, 14.1%; priority 1), cardiac and/or respiratory arrest (n=29, 11.4%; priority 1), cardiogenic shock and heart failure (n=25, 9.8%; priority 1), neurological diseases such as seizures (priority 1), demyelinating diseases and stroke (n=15, 5.9%; priority 1), trauma (n=10, 3.9%; priority 1), postoperative state (n=18, 7%; priority 1), and renal failure (n=9, 3.5%; priority 1) and were admitted for miscellaneous reasons (n=33, 12.9%; priority 1) (Table 3). Rates of neurological findings (62.5%), hypotension (82.5%), oliguria (47.5%), acidosis (92.5%), coagulopathy (30%), and cardiac arrest (45%) in children with early mortality were higher, while the probability of having fatal disease (52.5%) and chronic illness (75.6%) was lower (Table 2).
The median PICU LOS was 17 days (range, 1–205 days). The median duration of NIV was 1.55 days (range, 0–56 days), and the median duration of IMV was 15.85 days (range, 1–205 days). The most frequently used inotropes were adrenaline only (9.8%) and adrenaline, noradrenaline, and dopamine (26%). The median VIS was 14 points, (range, 10–24 points) and 67 patients (26.3%) needed CRRT, 14 patients (5.5%) needed PEX, 51 patients (20%) needed ECMO (45 veno-arterial [VA] ECMO, 6 veno-venous [VV] ECMO), and 10 patients needed extracorporeal cardiopulmonary resuscitation. A central line was placed in 94.1% of patients and a chest tube was inserted in 9.4%. While 38.6% of cases needed surgery, 21.7% had pulmonary bleeding; additionally, after admission to intensive care, 47.2% had NIs and 14.9% had intracranial bleeding. Invasive procedures performed on patients and the complications that occurred are reported in Table 4.
While the need for PEX (0%) and CRRT (7.5%) in patients who died early was lower than in children who died later, the need for VV-ECMO and VA-ECMO was similar (2.5%, 2.5%, and 2.2% vs. 17.5%, 15.1%, and 19.2%) (Table 2, Figure 3B). Blood and/or urine cultures were obtained from all patients. Infection was detected in 141 patients (55.5%), with 120 (47.2%) being hospital-associated and 26 (10.2%) being community-acquired. Heart disease (25.1%), malignancy (23.2%), multiple illnesses (11%), and neurological disease (11%) were the leading causes of death (Table 4). Surgical complications (10%), multi-organ failure (27.5%), neurological disease (22.5%), and heart disease (72.5%) were prominent in patients who died early in comparison to those that died late (Figure 3C).
Acute critically ill cases may experience respiratory failure necessitating MV, shock, extensive coagulopathy, and other organ failures, thus requiring ICU admission. [6]. Mortality in the PICU depends on a variety of factors, such as underlying chronic illnesses, NIs, the primary reasons for admission to the PICU, LOS in the PICU, and surgical complications. Understanding early death trends and the characteristics of children who die early is crucial to obtaining a rapid and targeted clinical trial record before death. Since our hospital does not have a palliative care unit in pediatric services, terminal patients are followed in the ICU.
The mean age of patients was 64.3 months in this study, whereas, in the 6-year report by Ayar et al. [7], the mean age was 93.1 months. Existing literature suggests that most PICU nonsurvivors are 1–5 years of age. Forty patients (15.7%) died early in this study. The median age of children who died early was higher at 81.8 months (range, 2–248 months). In a previous study, it was reported that the rate of patients who died early was 15% and the mean age of early death patients was 43.1 months (range, 10.3–119.3 months) [8]. The early death rate in this study was found to be comparable to other reports in the literature.
Most of the 254 nonsurvivors (83.9%) had underlying chronic illnesses, whereas, in a similar report by Poyrazoğlu et al. [9], 47.2% of nonsurvivors had chronic illnesses. In the report by Edwards et al. [10], 74% of the nonsurvivors had complex chronic conditions. The hospital is a tertiary care center, which explains the prevalence of chronic illness in nonsurvivors. The organization of palliative care centers for such patients may facilitate more efficient use of PICU resources.
The most common comorbidity in our patients was heart disease (25.1%). Studies report malignancy and neurologic and metabolic disorders as the most common underlying illnesses in patients that died in the PICU [11,12]. Our hospital is a referral hospital for pediatric cardiac patients where heart transplantation, ECMO, and other mechanical circulatory support can be offered, which accounts for the fact that most deaths occur in cardiac patients. Other common underlying illnesses in our PICU nonsurvivors were malignancy (23.2%) and neurologic disorders (11%). Most nonsurvivors presented to the emergency room (21.6%) or were admitted through the emergency medical transfer system (22.4%). In a 2-year retrospective study of mortality in the PICU, 49% of patients presented to the emergency room and 51% of the patients were transferred by ambulance during the first year, whereas, in the second year, 52% of patients presented to the emergency room and 48% were transferred by ambulance [13].
The most common causes of admission to the PICU in our study were respiratory failure (29.9%), sepsis (14.1%), and cardiac arrest (11.4%). Kılıç et al. [14] reported that 24.2% of their nonsurvivors were admitted due to respiratory failure, Ayar et al. [7] found that 24.5% of nonsurvivors were admitted due to respiratory failure, 15.5% were admitted for infections, and 13% were admitted for hematologic disease. Similar to existing literature that states respiratory failure and sepsis are the most common reasons for PICU admission in nonsurvivors, the most common causes for PICU admission of nonsurvivors in our study were respiratory failure and sepsis, which ultimately have effects on mortality.
Nonsurvivors commonly had hypoglycemia (68.5%), neurologic symptoms (43.5%), and acidosis (78.3%) at admission. Hematologic changes, rhythm disorders, renal failure, and NIs have been linked with mortality in a number of studies [15,16]. However, a correlation between mortality and findings on admission has not been adequately studied in the literature, which is why we consider our study to be an important piece of work in this regard. In addition, while multi-organ failure and cardiac diseases are common causes in patients who die early, sepsis and septic shock as causes have been reported in another study [8]. The reason our study differs from the literature may be that cardiac patients are followed up with frequently at our center.
The median LOS of PICU for nonsurvivors was 17 days (range, 1–205 days), which was longer in comparison to other studies. Moynihan et al. [13] reported that the median number of ICU stays of 2,672 patients who died was 3.4 days (range, 1.2–10 days), and 860 (32.2%) of these patients were admitted to the ICU for ≥7 days [12]. Most nonsurvivors (83.8%) with a long LOS in the PICU in our center had chronic cardiac and neurologic illnesses. Heart and liver transplants are also performed in our center, which may have contributed to the seemingly excessive LOS of PICU in the study.
NIV was the initial mode of support in 23.2% of patients, while 76.8% received IMV on admission. Patients who worsened on NIV were ventilated invasively. The median duration of NIV support was 1.55 days (range, 0–56 days) and the median duration of IMV was 15.85 days (range, 1–205 days). Complications of MV occurred in 0.8% of patients. Complications of MV were previously reported to occur in 20%–64% of patients, with 25.6% having bilateral lung disease and 21.6% having cyanotic heart disease [17]. Poyrazoğlu et al. [9] reported a median duration of MV of 3.5 days (range, 0.1–145 days), while Moynihan et al. [13] reported a median duration of MV of 2.6 days (range, 1–8.7 days) [18]. The longer duration of MV can be accounted for by the nature of the patients, who required advanced therapeutic interventions such as ECMO, high-frequency ventilation, and left and right ventricular mechanical support.
NIs, one of the most prevalent problems in ICUs, are associated with increases in the LOS, morbidity, and mortality. Becerra et al. [18] reported a 20% incidence of NIs. Risk factors for NIs include underlying chronic ailments, the presence of indwelling central lines, surgery, transfusion, sedation, parenteral nutrition, long hospitalization, and long duration of MV. Most common infections are related to the use of central lines, ventilators, and urinary catheters [18]. A multi-center study in Europe has reported a 23.6% incidence of NIs, with pneumonia being the most common type [19]. NIs occurred in 141 patients (55.5%) in our study, and 16 patients (10.2%) had community-acquired infections. These patients had underlying chronic illnesses and many had previous hospitalizations and lengthy periods of hospitalization, which account for the high rate of NIs in the study. Common causes of death in our study were cardiac disease (25.1%), hematologic/oncologic disease (23.2%), multiple illnesses (11%), and neurologic illness (11%). Moynihan et al. [13] report that 50% of their nonsurvivors had congenital anomalies, 14% had hemato-oncologic illness, and 11% had cardiac disease, whereas, in the study by Naghib et al. [20], 28% of nonsurvivors had congenital anomalies and 28% had cardiac disease. Sands et al. [21] reported that 19.6% of their patients died of infections or trauma, 17.2% died of congenital anomalies, and 16.2% succumbed to malignancy. Cardiac disease was very prevalent in this study because mechanical ventilatory support is routinely provided in our center both for cardiac disease and cardiorespiratory deterioration due to any cause. CRRT was prescribed to 26.4% of patients, while 5.5% needed PEX, 20% needed ECMO, and 10 patients needed extracorporeal cardiopulmonary resuscitation. Moynihan et al. [13] reported that 17.9% of their nonsurvivors needed CRRT, while Pollack et al. [22] reported that 16.1% of their nonsurvivors needed ECMO support and 2.7% of nonsurvivors needed CRRT and PEX [13]. The prevalent need for extracorporeal organ support in this study is thus in keeping with existing reports.
The median VIS was 14 points (range, 10–24 points) in this study. Haque et al. [23] reported that mortality for patients with a VIS <20 points was 38.9%, while that for patients with a VIS >20 points was 100%. Davidson et al. [24] suggest that VIS can be used to quantify the need for hemodynamic support during the first 48 hours of admission. In this study, the data also suggest that a high VIS is associated with mortality. In the ICU, the frequent use of extracorporeal treatments and infection control in recent years has led to a significant decrease in the mortality rate (Figure 1). In this study, the 5-year mortality rate was 9.1%. A study of PICU mortality in 2005 revealed a mortality rate of patients admitted to intensive care, while in the study by Kilic et al. [14], the mortality rate was 15.6%. The literature suggests that the overall PICU mortality rate is 4% in the United States [8], 5.6% in Europe, and 4% in Australia [25], respectively. Our mortality rate was greater than in reports from developed countries but lower than in those from developing countries. If end-stage or irreversible cases were omitted from the data, the overall mortality rate would be lower. The retrospective single-center study design, lack of data on morbidities, and lack of information on the time of day when death occurred are the main limitations of this study.
PICUs provide continuous multidisciplinary care for a broad array of pediatric diseases. Invasive infections, chronic diseases, and the spectrum of septic shock are important causes of mortality in the PICU. We found that rates of neurological findings, hypotension, oliguria, acidosis, coagulation disorders, and cardiac arrest as well as PRISM III scores were higher in children who died early compared to those who died later. ECMO was commonly provided during the 5-year study period in our center, which was immensely helpful in keeping certain patient populations alive.
▪ Invasive infections, chronic diseases, and the spectrum of septic shock are important causes of mortality in pediatric intensive care unit.
▪ Understanding early death trends and characteristics of children who die early is crucial to obtaining a rapid and targeted clinical trial record before death.
▪ We found that rates of neurological findings, hypotension, oliguria, acidosis, coagulation disorder, and cardiac arrest and Pediatric Risk of Mortality III scores were higher in children who died early compared to those who died later.
REFERENCES
1. Pearson G, Barry P, Timmins C, Stickley J, Hocking M. Changes in the profile of paediatric intensive care associated with centralisation. Intensive Care Med. 2001; 27:1670–3.

2. González-Cortés R, López-Herce-Cid J, García-Figueruelo A, Tesorero-Carcedo G, Botrán-Prieto M, Carrillo-Álvarez A. Prolonged stay in pediatric intensive care units: mortality and healthcare resource consumption. Med Intensiva. 2011; 35:417–23.

3. Valli G, Galati E, De Marco F, Bucci C, Fratini P, Cennamo E, et al. In-hospital mortality in the emergency department: clinical and etiological differences between early and late deaths among patients awaiting admission. Clin Exp Emerg Med. 2021; 8:325–32.

4. Levin M, Quint PA, Goldstein B, Barton P, Bradley JS, Shemie SD, et al. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: a randomised trial. Lancet. 2000; 356:961–7.

5. Nates JL, Nunnally M, Kleinpell R, Blosser S, Goldner J, Birriel B, et al. ICU admission, discharge, and triage guidelines: a framework to enhance clinical operations, development of ınstitutional policies, and further research. Crit Care Med. 2016; 44:1553–602.
6. Zhang JJ, Cao YY, Tan G, Dong X, Wang BC, Lin J, et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy. 2021; 76:533–50.

7. Ayar G, Uysal Yazici M, Sahin S, Gunduz RC, Yakut HI, Oden Akman A, et al. Six year mortality profile of a pediatric ıntensive care unit: associaton between out-of-hours and mortality. Arch Argent Pediatr. 2019; 117:120–5.
8. Johnson KT, Görges M, Murthy S. Characteristics and timing of mortality in children dying with ınfections in North American PICUs. Pediatr Crit Care Med. 2021; 22:365–79.
9. Poyrazoğlu H, Dursun İ, Güneş T, Akçakuş M, Konuşkan B, Canpolat M, et al. Evaluation and outcome analysis of patients in pediatric ıntensive care unit. Erciyes Med J. 2008; 30:232–7.
10. Edwards JD, Houtrow AJ, Vasilevskis EE, Rehm RS, Markovitz BP, Graham RJ, et al. Chronic conditions among children admitted to U.S. pediatric intensive care units: their prevalence and impact on risk for mortality and prolonged length of stay. Crit Care Med. 2012; 40:2196–203.
11. Fraser LK, Parslow R. Children with life-limiting conditions in paediatric intensive care units: a national cohort, data linkage study. Arch Dis Child. 2018; 103:540–7.

12. Ghaffari J, Abbaskhanian A, Nazari Z. Mortality rate in pediatric intensive care unit (PICU): a local center experience. int J Pediatr. 2014; 2:81–8.
13. Moynihan KM, Alexander PM, Schlapbach LJ, Millar J, Jacobe S, Ravindranathan H, et al. Epidemiology of childhood death in Australian and New Zealand intensive care units. Intensive Care Med. 2019; 45:1262–71.

14. Kiliç FZ, Çoban Y, Davutoglu M, Dalkiran T. A retrospective analysis of patients monitored in a pediatric ıntensive care unit and an ınvestigation of factors affecting mortality. J Pediatr Emerg Intensive Care Med. 2016; 3:140–5.
15. Bailey D, Phan V, Litalien C, Ducruet T, Mérouani A, Lacroix J, et al. Risk factors of acute renal failure in critically ill children: a prospective descriptive epidemiological study. Pediatr Crit Care Med. 2007; 8:29–35.

16. Goodman S, Shirov T, Weissman C. Supraventricular arrhythmias in intensive care unit patients: short and long-term consequences. Anesth Analg. 2007; 104:880–6.

17. Khemani RG, Markovitz BP, Curley MA. Characteristics of children intubated and mechanically ventilated in 16 PICUs. Chest. 2009; 136:765–71.

18. Becerra MR, Tantaleán JA, Suárez VJ, Alvarado MC, Candela JL, Urcia FC. Epidemiologic surveillance of nosocomial infections in a pediatric ıntensive care unit of a developing country. BMC Pediatr. 2010; 10:66.
19. Raymond J, Aujard Y; European Study Group. Nosocomial infections in pediatric patients: a European, multicenter prospective study. Infect Control Hosp Epidemiol. 2000; 21:260–3.

20. Naghib S, van der Starre C, Gischler SJ, Joosten KF, Tibboel D. Mortality in very long-stay pediatric intensive care unit patients and incidence of withdrawal of treatment. Intensive Care Med. 2010; 36:131–6.

21. Sands R, Manning JC, Vyas H, Rashid A. Characteristics of deaths in paediatric intensive care: a 10-year study. Nurs Crit Care. 2009; 14:235–40.

22. Pollack MM, Banks R, Holubkov R, Meert KL; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Morbidity and mortality in critically ill children: I. Pathophysiologies and potential therapeutic solutions. Crit Care Med. 2020; 48:790–8.

23. Haque A, Siddiqui NR, Munir O, Saleem S, Mian A. Association between vasoactive-inotropic score and mortality in pediatric septic shock. Indian Pediatr. 2015; 52:311–3.

24. Davidson J, Tong S, Hancock H, Hauck A, da Cruz E, Kaufman J. Prospective validation of the vasoactive-inotropic score and correlation to short-term outcomes in neonates and infants after cardiothoracic surgery. Intensive Care Med. 2012; 38:1184–90.

25. Köroğlu TF, Çıtak A, Bayrakçı B, Kendirli T, Yıldızdaş D, Karaböcüoğlu M. Pediatric intensive care services in Turkey, current situation and recommendations 2006. Pediatric Emergency Medicine and Intensive Care Association; 2016 [cited 2022 Nov 3]. Avaible from: http://www.cayd.org.tr/images/userFiles/Documents/.
Figure 2.
(A) Numbers of admissions and nonsurvivors annually from 2015 to 2019. (B) Annual mortality rates.
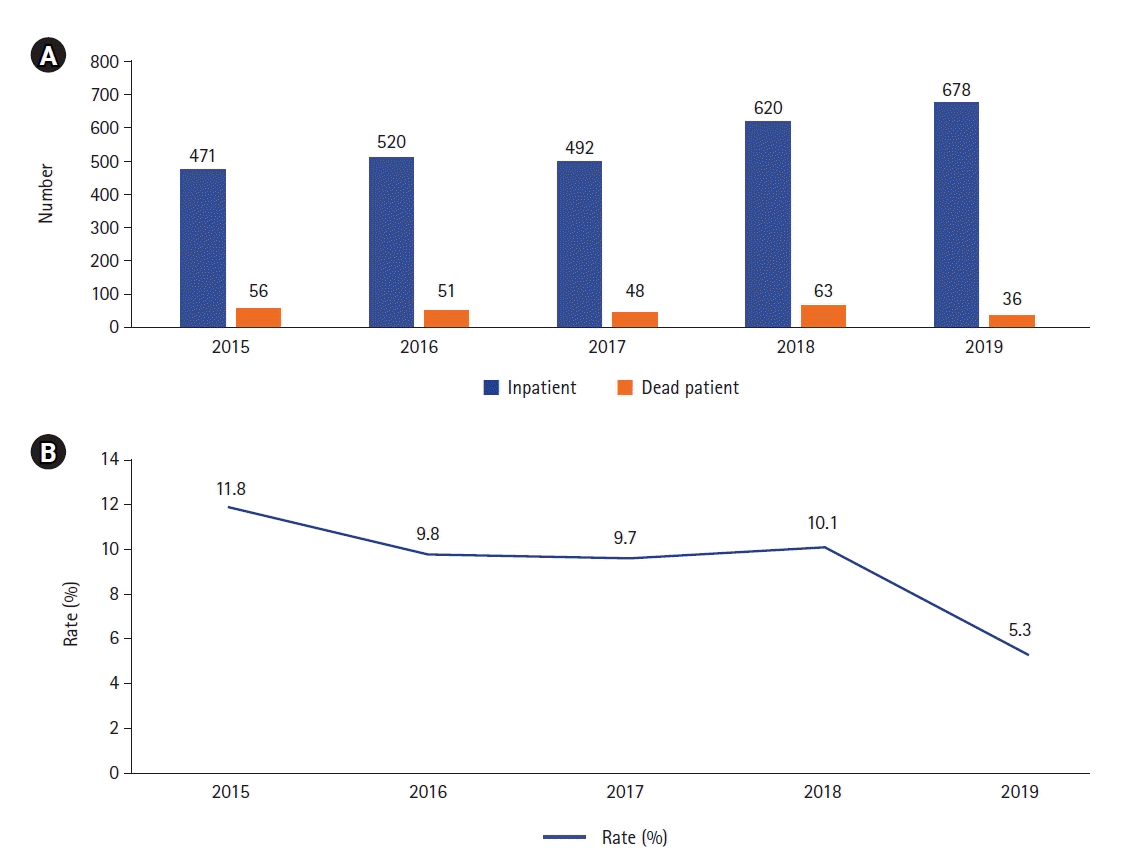
Figure 3.
(A) Procedures based on timing of death, including extracorporeal membrane oxygenation (ECMO; veno-venous [VV] or veno-arterial [VA]), plasma exchange (PEX), and continuous renal replacement therapy (CRRT) (≤1 or ≥2 day). (B) Location prior to pediatric intensive care unit (PICU) admission based on timing of death, including from the institution’s own emergency department or operating room and transfer from other hospitals. Patients transported from the neonatal ICU and pediatric wards of the hospital. (C) Diagnosis based on timing of death in the PICU.
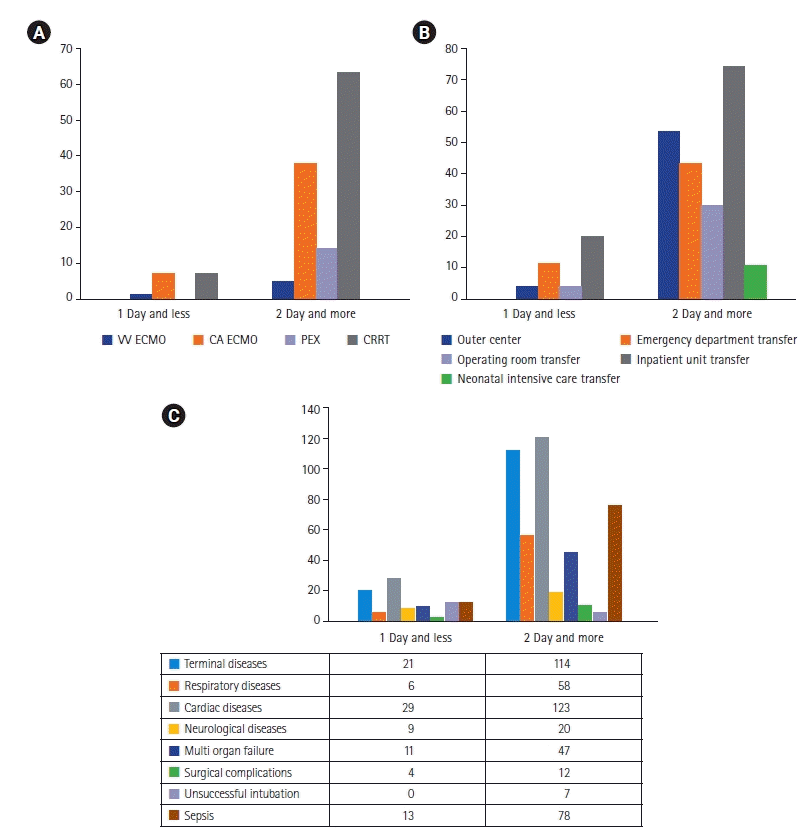
Table 1.
Demographic features of nonsurvivors
Table 2.
Clinical characteristics of patients according to the day of death
Values are presented as median (interquartile range) or number (%).
PRISM: Pediatric Risk of Mortality; PICU: pediatric intensive care unit; ED: emergency department; NICU: neonatal intensive care unit; VV: veno-venous; ECMO: extracorporeal membrane oxygenation; VA: veno-arterial; CRRT: continuous renal replacement therapy; PEX: plasma exchange.
Table 3.
Hospitalization indications in total study group
Table 4.
Invasive procedures applied to patients who died, complications during hospitalization, and causes of death




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download