1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382:1708–20.

2. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020; 395:1763–70.

3. ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012; 307:2526–33.
4. Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020; 46:1099–102.

5. Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020; 46:854–87.

6. Qadri SK, Ng P, Toh TS, Loh SW, Tan HL, Lin CB, et al. Critically ill patients with COVID-19: a narrative review on prone position. Pulm Ther. 2020; 6:233–46.

7. Venus K, Munshi L, Fralick M. Prone positioning for patients with hypoxic respiratory failure related to COVID-19. CMAJ. 2020; 192:E1532–7.

8. Vignon P, Evrard B, Asfar P, Busana M, Calfee CS, Coppola S, et al. Fluid administration and monitoring in ARDS: which management? Intensive Care Med. 2020; 46:2252–64.

9. Grissom CK, Hirshberg EL, Dickerson JB, Brown SM, Lanspa MJ, Liu KD, et al. Fluid management with a simplified conservative protocol for the acute respiratory distress syndrome. Crit Care Med. 2015; 43:288–95.

10. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006; 354:2564–75.

11. Jozwiak M, Teboul JL, Monnet X. Extravascular lung water in critical care: recent advances and clinical applications. Ann Intensive Care. 2015; 5:38.

12. Monnet X, Anguel N, Osman D, Hamzaoui O, Richard C, Teboul JL. Assessing pulmonary permeability by transpulmonary thermodilution allows differentiation of hydrostatic pulmonary edema from ALI/ARDS. Intensive Care Med. 2007; 33:448–53.

13. Groeneveld AB, Verheij J. Extravascular lung water to blood volume ratios as measures of permeability in sepsis-induced ALI/ARDS. Intensive Care Med. 2006; 32:1315–21.

14. Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 2020; 295:202–7.

15. Fan E, Beitler JR, Brochard L, Calfee CS, Ferguson ND, Slutsky AS, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020; 8:816–21.

16. Monnet X, Teboul JL. Transpulmonary thermodilution: advantages and limits. Crit Care. 2017; 21:147.

17. Gattinoni L, Busana M, Giosa L, Macrì MM, Quintel M. Prone positioning in acute respiratory distress syndrome. Semin Respir Crit Care Med. 2019; 40:94–100.

18. Grieco DL, Bongiovanni F, Chen L, Menga LS, Cutuli SL, Pintaudi G, et al. Respiratory physiology of COVID-19-induced respiratory failure compared to ARDS of other etiologies. Crit Care. 2020; 24:529.

19. Boerma EC, Bethlehem C, Stellingwerf F, de Lange F, Streng KW, Koetsier PM, et al. Hemodynamic characteristics of mechanically ventilated COVID-19 patients: a cohort analysis. Crit Care Res Pract. 2021; 2021:8882753.

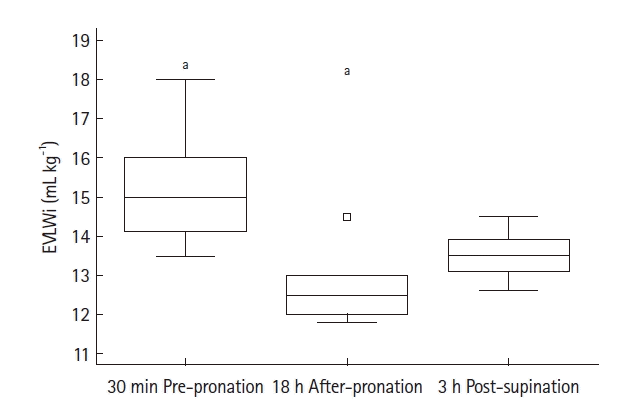
20. Rasch S, Schmidle P, Sancak S, Herner A, Huberle C, Schulz D, et al. Increased extravascular lung water index (EVLWI) reflects rapid non-cardiogenic oedema and mortality in COVID-19 associated ARDS. Sci Rep. 2021; 11:11524.

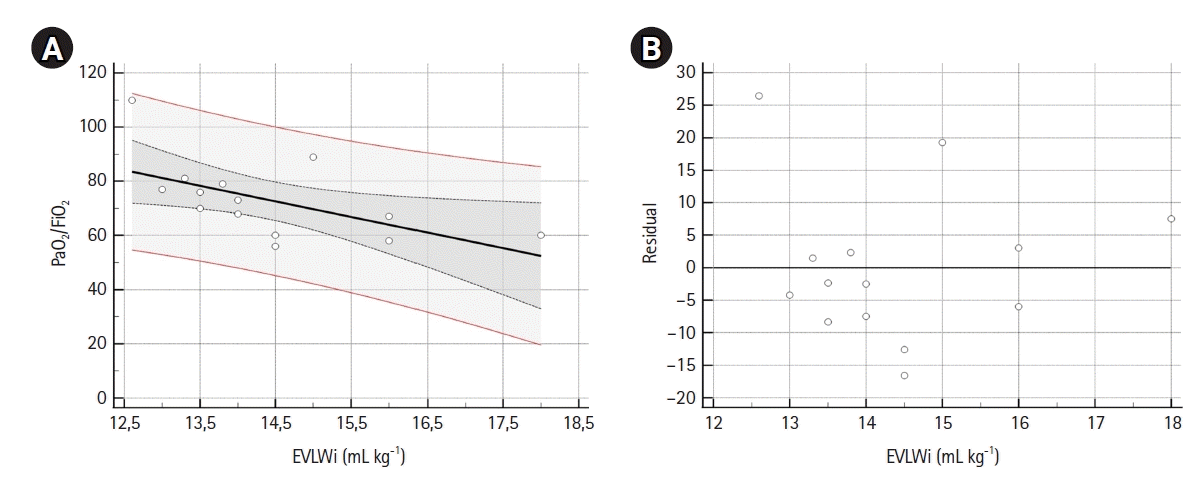
21. Shi R, Lai C, Teboul JL, Dres M, Moretto F, De Vita N, et al. COVID-19 ARDS is characterized by higher extravascular lung water than non-COVID-19 ARDS: the PiCCOVID study. Crit Care. 2021; 25:186.

22. Asar S, Acicbe Ö, Sabaz MS, Tontu F, Canan E, Cukurova Z, et al. Comparison of respiratory and hemodynamic parameters of COVID-19 and non-COVID-19 ARDS patients. Indian J Crit Care Med. 2021; 25:704–8.

23. McAuley DF, Giles S, Fichter H, Perkins GD, Gao F. What is the optimal duration of ventilation in the prone position in acute lung injury and acute respiratory distress syndrome? Intensive Care Med. 2002; 28:414–8.

24. Ruste M, Bitker L, Yonis H, Riad Z, Louf-Durier A, Lissonde F, et al. Hemodynamic effects of extended prone position sessions in ARDS. Ann Intensive Care. 2018; 8:120.

25. Tarragón B, Valdenebro M, Serrano ML, Maroto A, Llópez-Carratalá MR, Ramos A, et al. Acute kidney failure in patients admitted due to COVID-19. Nefrologia (Engl Ed). 2021; 41:34–40.

26. Sweeney DA, Malhotra A. Coronavirus disease 2019 respiratory failure: what is the best supportive care for patients who require ICU admission? Curr Opin Crit Care. 2021; 27:462–7.

27. Kushimoto S, Endo T, Yamanouchi S, Sakamoto T, Ishikura H, Kitazawa Y, et al. Relationship between extravascular lung water and severity categories of acute respiratory distress syndrome by the Berlin definition. Crit Care. 2013; 17:R132.

28. Bhattacharjee A, Pradhan D, Bhattacharyya P, Dey S, Chhunthang D, Handique A, et al. How useful is extravascular lung water measurement in managing lung injury in intensive care unit? Indian J Crit Care Med. 2017; 21:494–9.

29. Osuchowski MF, Winkler MS, Skirecki T, Cajander S, Shankar-Hari M, Lachmann G, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021; 9:622–42.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download