Abstract
Five-FU is a potent chemotherapeutic agent for suppressing endothelial cell growth. The purpose of this study was to investigate the usefulness of local peritumor injection of 5-FU for patients with advanced gastric cancer (AGC) for the prevention of anemia. Between January 2020 and January 2022, patients aged 18 years or older with AGC and moderate anemia were included. A total of 200 mg of 5-FU was injected per session at ten points of the lesion (20 mg at each point) every 7 days for 4 to 12 weeks. Patients received a blood test for toxicity at every cycle. From one of these patients, endoscopic biopsy specimens were taken from gastric cancer before and after injecting 5-FU for immunostaining. A total of five AGC patients participated in this study. For most patients, hemoglobin levels were maintained without transfusions during 5-FU injection, and expression levels of thrombospondin-1 was increased after injection compared to those before injection. Blood test results during 5-FU injection showed no significant change in serum glutamic oxalacetic transaminase/glutamic pyruvic transaminase, total bilirubin, or creatinine level. The results of this study showed the possibility of local peritumor 5-FU injection as a treatment for relieving anemia of patients with gastric cancer.
Gastric cancer is the second most common cancer in Korea.1 Although treatment outcomes have improved significantly compared to the past due to early diagnosis, in many cases, the diagnosis is delayed with the disease progressing to advanced gastric cancer (AGC). Anemia is one of the most common problems of patients with AGC.2 Gastric cancer is associated with chronic blood loss, poor nutrition, chemotherapy, and surgical treatment that results in inhibition of iron absorption, all of which can synergistically increase the risk for iron deficiency anemia.3 Anemia in cancer patients requires special attention because it is related to a poor prognosis,4 physical symptoms,5 poor performance status6, and poor quality of life (QoL).7
Current treatment modalities for anemia of patients with gastric cancer include red blood cells (RBC) transfusion, palliative radiation therapy with or without chemotherapy, endoscopic treatment, and intravascular embolization. RBC transfusions are the fastest and easiest way to remove symptoms caused by anemia. Radiation therapy has been reported to be adequate to control bleeding from gastric cancer.8,9 Intravascular embolization also can be used in well-selected patients.10 However, transfusions are also associated with infections, transfusion reactions, and alloimmunization.11 These treatments may require bleeding to occur. Thus, bleeding cannot be prevented. In addition, time and manpower are required to prepare these treatments.
Therefore, an alternative treatment method is needed to alleviate symptoms and improve the QoL of long-term survival patients with anemia. Local injection of anticancer drugs could be one possible method.12,13 In a previous experimental study, when 5-FU (250 mg), an anticancer drug, was locally injected into the marginal area of the tumor at intervals of 1 week for four weeks in patients with AGC, the size of the tumor was reduced by 30-40% without specific side effects.14 Five-FU is the most potent chemotherapeutic for suppressing endothelial cell growth.15 It can inhibit angiogenesis by inducing thrombospondin-1 (TSP-1) expression.16 The purpose of this study was to investigate the usefulness of local injection of 5-FU for patients with AGC for the prevention of anemia.
Between January 2020 to January 2022, patients aged 18 years or older with AGC and moderate anemia2 were included. Eligibility criteria were: 1) histologically confirmed gastric adenocarcinoma; 2) unresectable or recurrent disease; 3) adequate hematological function (a white blood cell count between 3,000 and 12,000/mm3, a platelet count ≥ 100,000/mm3), adequate hepatic function (serum total bilirubin level of ≤2.0 mg/dL, serum glutamic oxalacetic transaminase (SGOT)/glutamic pyruvic transaminase (SGPT) levels ≤100 IU/L), adequate renal function (serum creatinine level ≤1.5 mg/dL), serum C-reactive protein level ≤10 mg/dL, and written informed consent; and (4) Eastern Cooperative Oncology Group performance 0-2.17
Exclusion criteria were marked pleural effusion or ascites, active infection, severe comorbidity such as heart disease or renal disease, metastasis to the central nervous system, mental disorder, active concomitant malignancy, and pregnant or nursing women and women of childbearing age unless they were practicing effective contraception.
All patients provided written informed consent for receiving chemotherapy. This study was reviewed and approved by the Ministry of Food and Drug Safety and Institutional Reviewed Board of Kangbuk Samsung Hospital (KBSMC2019-09-021).
A total of 200 mg of 5-FU was injected per session at ten points of the lesion (20 mg at each point) every 7 days for 4 to 12 weeks (Fig. 1). Patients received a blood test for toxicity every cycle. From one of these patients, endoscopic biopsy specimens were taken from gastric cancer before and after injecting 5-FU for immunostaining.
Patients’ records were reviewed from the time point 30 days before and after initiating injection until their death. Blood tests were performed the day before drug administration and on the day of administration at weekly intervals. In addition, the same test was performed weekly or every 2 weeks at one month after the end of treatment. Blood tests performed were as follows: white blood cell (WBC), hemoglobin, platelet count, WBC differential count (neutrophil, lymphocyte, monocyte, eosinophil, basophil), albumin, total bilirubin, AST, ALT, gamma-glutamyl transpeptidase, BUN and creatinine. The amount of blood transfused and change of body weight were evaluated. Factors that might affect anemia levels, including chemotherapy, drugs, and comorbidities, were investigated.
Sections (4 μm in thickness) were cut from each tissue array block. They were then deparaffinized and dehydrated. Immunohistochemical staining was performed using an automatic immunostainer (BenchMark® XT; Ventana Medical Systems Inc., Tucson, AZ, USA) according to the manufacturer’s instructions. Primary antibodies used were anti-TSP-1 (sc-59887), anti-vascular endothelial growth factor (VEGF) (sc-7269) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-TSP-2 antibody (ab112543) (Abcam, Cambridge, UK).
Human gastric cancer cell (NCI-N87) was obtained from the Korean Cell Line Bank (Seoul, Korea) and cultured in Dulbecco’s modified Eagle medium (Thermo Fisher Scientific, Rockford, IL, USA) supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, and 2 mM L-glutamine at 37°C in an atmosphere of 5% CO2.
Cell extracts were lysed with RIPA buffer (25 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate) in the presence of Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific). Samples were subsequently centrifuged at 13,000 g for 20 minutes to pellet the cell debris. The concentrations of the proteins in the cell lysates were determined using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific), and bovine serum albumin was used as the standard. The proteins were resolved by electrophoresis on Bolt 4-12% Bis-Tris Plus gels (Thermo Fisher Scientific) and transferred onto polyvinylidene fluoride membranes (Thermo Fisher Scientific). The membranes were then blocked with Pierce Clear Milk Blocking Buffer (Thermo Fisher Scientific) for 1 hour and subsequently incubated overnight at 4°C with the primary antibodies against TSP-1, VEGF and β-actin. horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG antibodies (Santa Cruz Biotechnology, Dallas, TX, USA) were used as secondary antibodies. Immune complexes were detected using an ECL Prime Western Blotting Detection Reagent system (Amersham, Piscataway, NJ, USA).
All data are expressed as mean±SD. Hemoglobin level and transfusion volume at 1 month before drug infusion, during treatment, and after discontinuation of treatment were compared. Blood test results performed for safety were evaluated in the same way. Comparison between blood tests was performed using one-way analysis of variance on ranks and Wilcoxon signed rank test. The significance level was set at p<0.05. All analyses were performed using SPSS version 18 (IBM, Armonk, NY, USA).
A total of five AGC patients participated in the study (Table 1). All patients were accompanied by a persistent decrease in hemoglobin level. Two of them were patients who had a stent insertion due to gastric outlet obstruction. Two patients received four injections at weekly intervals. And the remaining three patients received 8, 10, and 12 injections, respectively (Fig. 2).
In most patients, levels of hemoglobin were maintained during 5-FU injection treatment without transfusion (Fig. 3). Only one patient received a blood transfusion once a month, but the amount of transfusion was significantly decreased compared to that before treatment. When transfusion volumes of all patients were compared before and during treatment, a significant decrease was seen after treatment (p<0.05) (Fig. 4).
Expression levels of TSP-1 in cancer tissues before and after 5-FU injection were compared using immunostaining. As shown in Fig. 5A, expression level of TSP-1 was increased after injection compared to those before injection. Western blot was performed in N87 human gastric ell line to confirm TSP-1 expression increase by 5-FU (Fig. 5B). The expression increased significantly in proportion to the concentration of 5-FU (p<0.05). However, the expression of VEGF after injection was not significantly different from that before injection.
Blood test results during 5-FU injection showed no significant change in SGOT/SGPT, total bilirubin, or creatinine level during treatments (p>0.05) (Fig. 6). In addition, there was no change in the patient's vital signs such as blood pressure, pulse, body temperature, and electrocardiogram, and no change were observed in blood and urine tests other than those mentioned above. Lastly, there were no subjective symptoms or systemic adverse events after 5-FU injection. However, one of the patients received percutaneous transhepatic biliary drainage (PTBD) treatment due to biliary obstruction in the last week of the injection, which resulted in significant changes in liver function tests. Thus, these results were not included in the safety assessment. Liver functions of this patient recovered after treatment.
The original purpose of this study was to determine whether 5-FU injection could reduce the size of the mass as in a previous paper14 or to determine whether injection of 5-FU along with a stent could inhibit re-obstruction when there was a local problem such as gastric outlet obstruction. However, in most patients with AGC, simple local 5-FU injection could not induce significant reduction in tumor size. Instead, anemia caused by bleeding in the tumor could be prevented.
In fact, it is well known that cardiotoxicity occurs as a rare side effect of 5-FU, which is often a limiting factor in the use of 5-FU in clinical oncology practice. A previous experimental study showed that very serious damage to endothelial cells, occasionally followed by platelet accumulation and fibrin formation, could be caused by direct toxicity of 5-FU.18 The mechanism for the prevention of anemia by 5-FU might be increased expression of TSP-1. TSP-1 is a potent endogenous inhibitor of angiogenesis. They can inhibit angiogenesis by directly affecting endothelial cell migration, proliferation, survival, and apoptosis and by antagonizing the activity of VEGF.19 TSP-1 can inhibit the release of VEGF from the extracellular matrix through suppression of matrix metalloproteinase-9 activity.20 In this study, expression levels of TSP-1 was significantly increased as a result of immunohistochemical staining. This result is consistent with those of a previous report showing that 5-FU induced TSP-1 in human colon carcinoma KM12C cells. A transcription factor, Egr-1, was also induced by 5-FU. It could bind to the promoter of TSP-1, enhancing its transcription with subsequent production of TSP-1 protein. TSP-1 expression was significantly up-regulated in xenograft tumor tissues and plasma in animals treated with S-1 at a sub-maximally tolerated dose.16
TSP-1 can antagonize VEGF in several important ways, including inhibition of VEGF release from the extracellular matrix, direct interaction, and inhibition of VEGF signal transduction. However, the expression of VEGF was not significantly affected in the immunostaining of this study. The possible explanation was that the injected 5-FU did not spread to the biopsy site because the biopsy was performed on the upper part of the tumor rather than the base of the 5-FU injection site. Another explanation was that peritumor injection of 5-FU might not have a significant effect on the expression of VEGF. In another study, although TSP-1 mRNA level was significantly induced by about 3-fold in the S-1 group, a significant change in VEGF mRNA expression was not seen in xenografts treated with S-1.16 Therefore, although 5-FU mediates TSP-1 on the expression of VEGF, the degree of its effect might not be so large.
Although it was difficult to prevent an increase in overall tumor size through 5-FU simple repeated injection, such injection could suppress the increase in local tumor size to some extent. Gastric outlet obstruction was present in two of five patients in the present study. Reocclusion was somewhat inhibited when 5-FU injection was used together with a stent. In one patient, the average albumin level was maintained at about 3.5 mg/dL during the injection for 2 and a half months. It showed a sharp decrease after stopping the injection. Albumin level in another patient was also maintained significantly for 2 months. However, it is difficult to say that these results are due to the effect of 5-FU injection treatment alone. This is because it is difficult to accurately evaluate the tumor size. The effect of stent insertion cannot be excluded. In addition, 5-FU injection could inhibit tissue fibrosis, thus inhibiting tumor-induced reduction in the size of the pyloric lumen. 5-FU is an antimetabolite, which interferes with RNA synthesis and consequently inhibits fibroblast proliferation. It also has an inhibitory effect on transforming growth factor-β1 induced expression of type I collagen gene. Clinically, 5-FU can be administered intralesionally for treating keloids. At a concentration of 50 mg/mL, it showed favorable results.
When change in tumor size was observed after peritumoral injection of 5-FU sol-gel using Pluronic F127 in an animal study, simple 5-FU injection did not induce a statistically significant change in size, consistent with results of our study. Injected 5-FU is quickly decomposed by dihydropyrimidine dehydrogenase without showing a significant anti-tumor effect. However, 5-FU sol-gel releases the drug slowly for at least 10 hours. Thus, it can have the same effect as continuous iv injection of the drug. There should be more such attempts in the future.
Another important point was that local injection of 5-FU at a high dose of 200 mg did not induce any side effects. Previously, an animal study was conducted to observe changes in anal pressure by local injection of sustained-release phenylephrine around anuses of rats. Although the injected phenylephrine dose reached several times the lethal dose, it did not induce significant changes in blood pressure or heart rate. Similarly, in this study, there was little change in patient's physical vital signs or blood tests, thus confirming its safety. However, in one patient, bilirubin and liver enzymes were elevated due to tumor-induced biliary obstruction, whereas these enzymes decreased after PTBD. These results were not included in the safety analysis of this study because it was not related to the drug.
In conclusion, peritumoral injection of 5-FU was not able to reduce tumor size or prevent metastasis in AGC, although it was able to significantly reduce the amount of transfusion by reducing bleeding from the tumor, which was thought to be an effect of inhibited angiogenesis due to increased expression levels of TSP-1. Future studies with more patients are needed.
REFERENCES
1. Jung KW, Park S, Kong HJ, et al. 2010; Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 25:1113–1121. DOI: 10.3346/jkms.2010.25.8.1113. PMID: 20676319. PMCID: PMC2908777.

2. Groopman JE, Itri LM. 1999; Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 91:1616–1634. DOI: 10.1093/jnci/91.19.1616. PMID: 10511589.

3. Bermejo F, García-López S. 2009; A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J Gastroenterol. 15:4638–4643. DOI: 10.3748/wjg.15.4638. PMID: 19787826. PMCID: PMC2754511.

4. Clarke H, Pallister CJ. 2005; The impact of anaemia on outcome in cancer. Clin Lab Haematol. 27:1–13. DOI: 10.1111/j.1365-2257.2004.00664.x. PMID: 15686502.

5. Ludwig H, Strasser K. 2001; Symptomatology of anemia. Semin Oncol. 28(2 Suppl 8):7–14. DOI: 10.1053/sonc.2001.25391. PMID: 11395846.

6. Ludwig H, Müldür E, Endler G, Hübl W. 2013; Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol. 24:1886–1892. DOI: 10.1093/annonc/mdt118. PMID: 23567147. PMCID: PMC3690908.

7. Holzner B, Kemmler G, Greil R, et al. 2002; The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol. 13:965–973. DOI: 10.1093/annonc/mdf122. PMID: 12123343.

8. Kondoh C, Shitara K, Nomura M, et al. 2015; Efficacy of palliative radiotherapy for gastric bleeding in patients with unresectable advanced gastric cancer: a retrospective cohort study. BMC Palliat Care. 14:37. DOI: 10.1186/s12904-015-0034-y. PMID: 26238344. PMCID: PMC4524128.

9. Asakura H, Hashimoto T, Harada H, et al. 2011; Palliative radiotherapy for bleeding from advanced gastric cancer: is a schedule of 30 Gy in 10 fractions adequate? J Cancer Res Clin Oncol. 137:125–130. DOI: 10.1007/s00432-010-0866-z. PMID: 20336314.

10. Pereira J, Phan T. 2004; Management of bleeding in patients with advanced cancer. Oncologist. 9:561–570. DOI: 10.1634/theoncologist.9-5-561. PMID: 15477642.

11. Aapro M, Österborg A, Gascón P, Ludwig H, Beguin Y. 2012; Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of i.v. iron. Ann Oncol. 23:1954–1962. DOI: 10.1093/annonc/mds112. PMID: 22575608.

12. Ohta H, Takagi K, Noguchi Y, et al. 1984; Endoscopic intramural injection of anti-neoplastic emulsion. Gan. 75:641–649.
13. Yamada K, Murphy GP, Douglass HO Jr. 1979; Comparison of intramural 5-fluorouracil and more conventional routes of drug administration on concentrations in gastric regional lymph nodes: a potential for trans-endoscopic adjuvant chemotherapy. J Surg Oncol. 11:341–349. DOI: 10.1002/jso.2930110409. PMID: 109704.

14. Robles-Jara C, Robles-Medranda C. 2010; Endoscopic chemotherapy with 5-fluorouracil in advanced gastric cancer. J Gastrointest Cancer. 41:75–78. DOI: 10.1007/s12029-009-9112-9. PMID: 19943195.

15. Zhang Y, Sun M, Huang G, et al. 2017; Maintenance of antiangiogenic and antitumor effects by orally active low-dose capecitabine for long-term cancer therapy. Proc Natl Acad Sci U S A. 114:E5226–E5235. DOI: 10.1073/pnas.1705066114. PMID: 28607065. PMCID: PMC5495268.

16. Ooyama A, Oka T, Zhao HY, Yamamoto M, Akiyama S, Fukushima M. 2008; Anti-angiogenic effect of 5-fluorouracil-based drugs against human colon cancer xenografts. Cancer Lett. 267:26–36. DOI: 10.1016/j.canlet.2008.03.008. PMID: 18420342.

17. Oken MM, Creech RH, Tormey DC, et al. 1982; Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 5:649–655. DOI: 10.1097/00000421-198212000-00014. PMID: 7165009.

18. Cwikiel M, Eskilsson J, Albertsson M, Stavenow L. 1996; The influence of 5-fluorouracil and methotrexate on vascular endothelium. An experimental study using endothelial cells in the culture. Ann Oncol. 7:731–737. DOI: 10.1093/oxfordjournals.annonc.a010723. PMID: 8905032.

19. Lawler PR, Lawler J. 2012; Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2:a006627. DOI: 10.1101/cshperspect.a006627. PMID: 22553494. PMCID: PMC3331684.

20. Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. 2001; Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 98:12485–12490. DOI: 10.1073/pnas.171460498. PMID: 11606713. PMCID: PMC60080.

Fig. 1
Schematic of 5-FU peritumor injection. A total of 200 mg of 5-FU was injected per session at ten points of the lesion (20 mg at each point) every 7 days for 4 to 12 weeks.
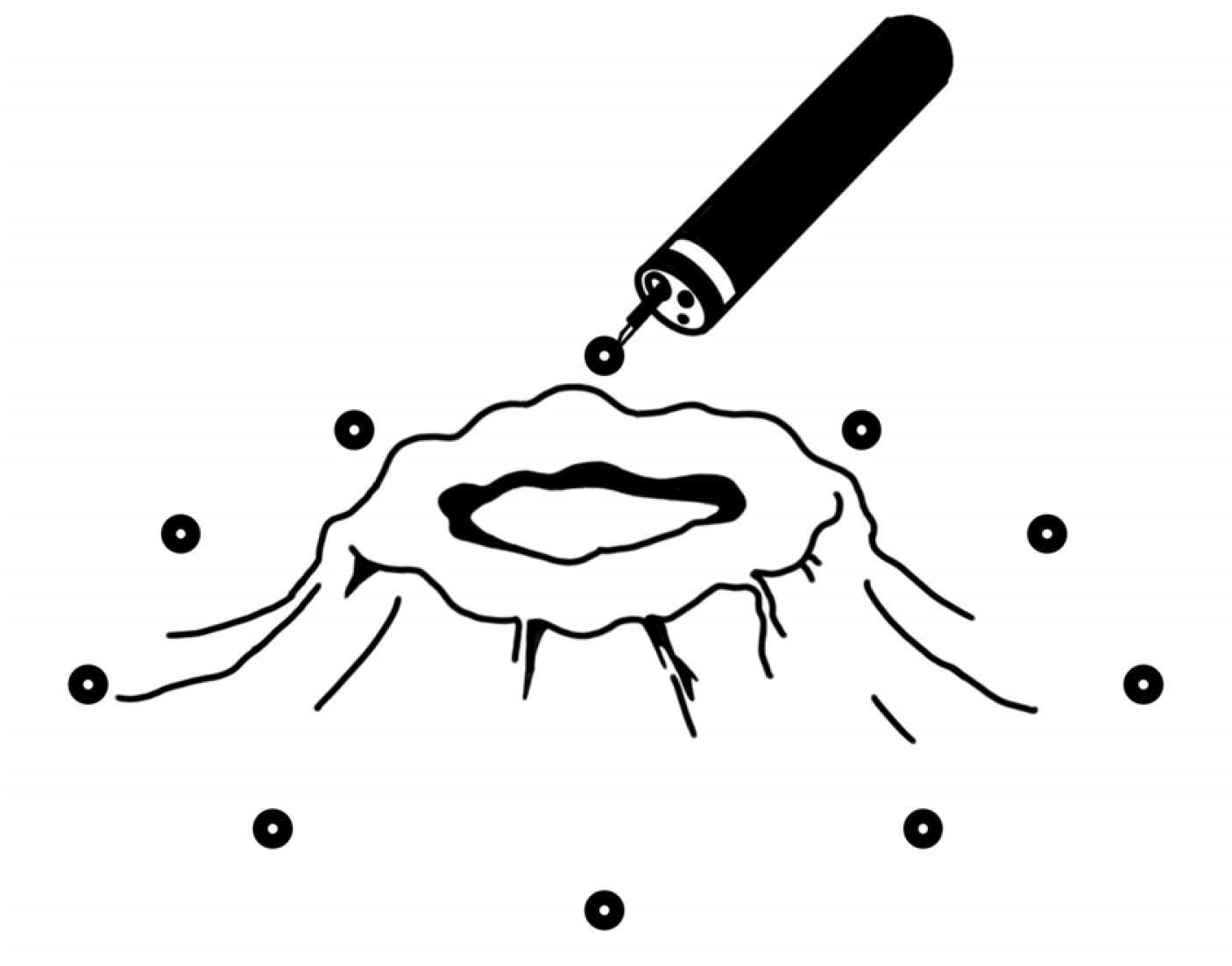
Fig. 2
Endoscopy pictures before and 1 month after peritumor 5-FU injection. Hyperemia of gastric mucosa was significantly reduced after 5-FU injection compared to before injection.
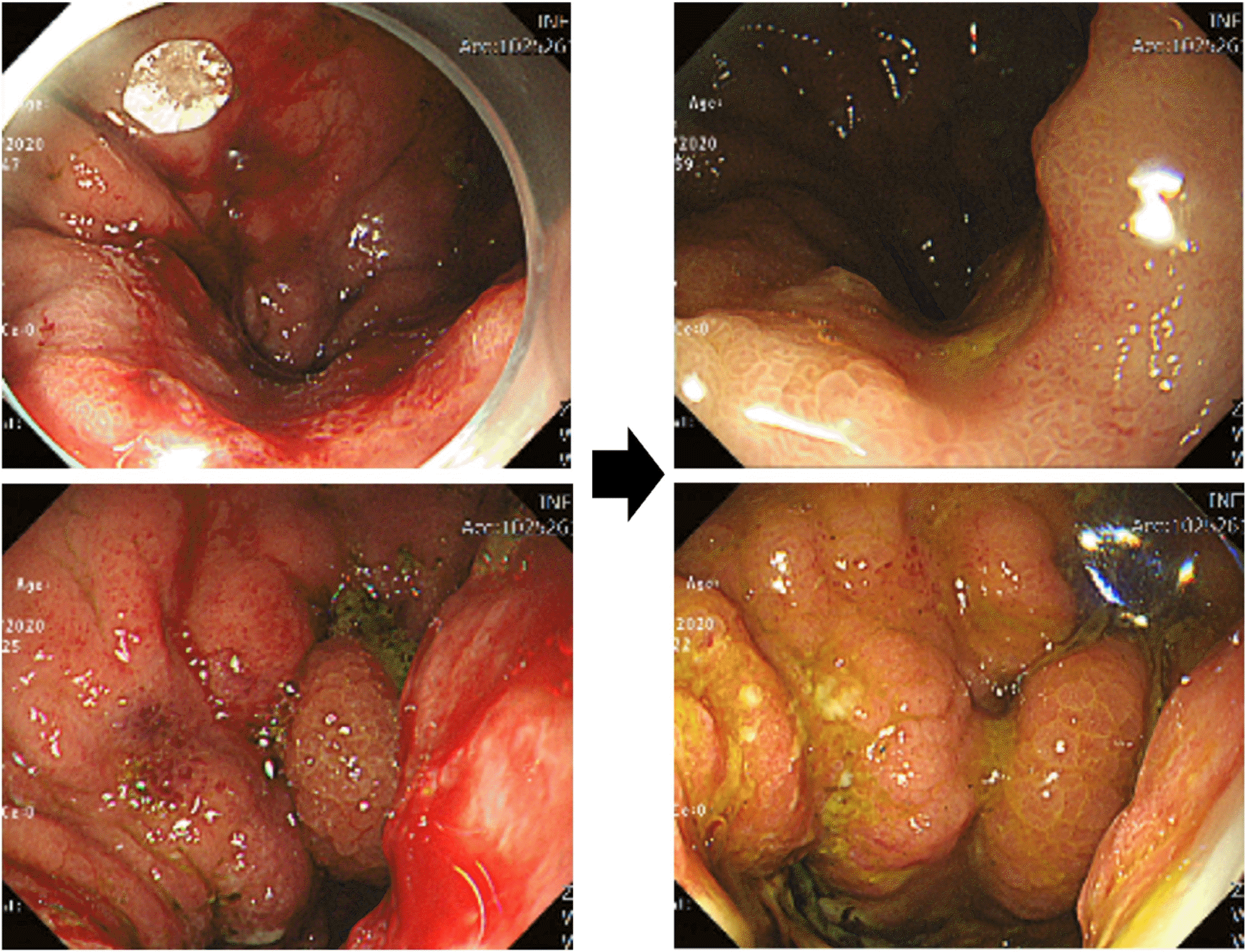
Fig. 3
(A-D) Comparison of hemoglobin level and transfusion volume before, during, and after treatment for each patient. In most patients, levels of hemoglobin were maintained during 5-FU injection treatment without transfusion. *p<0.05.
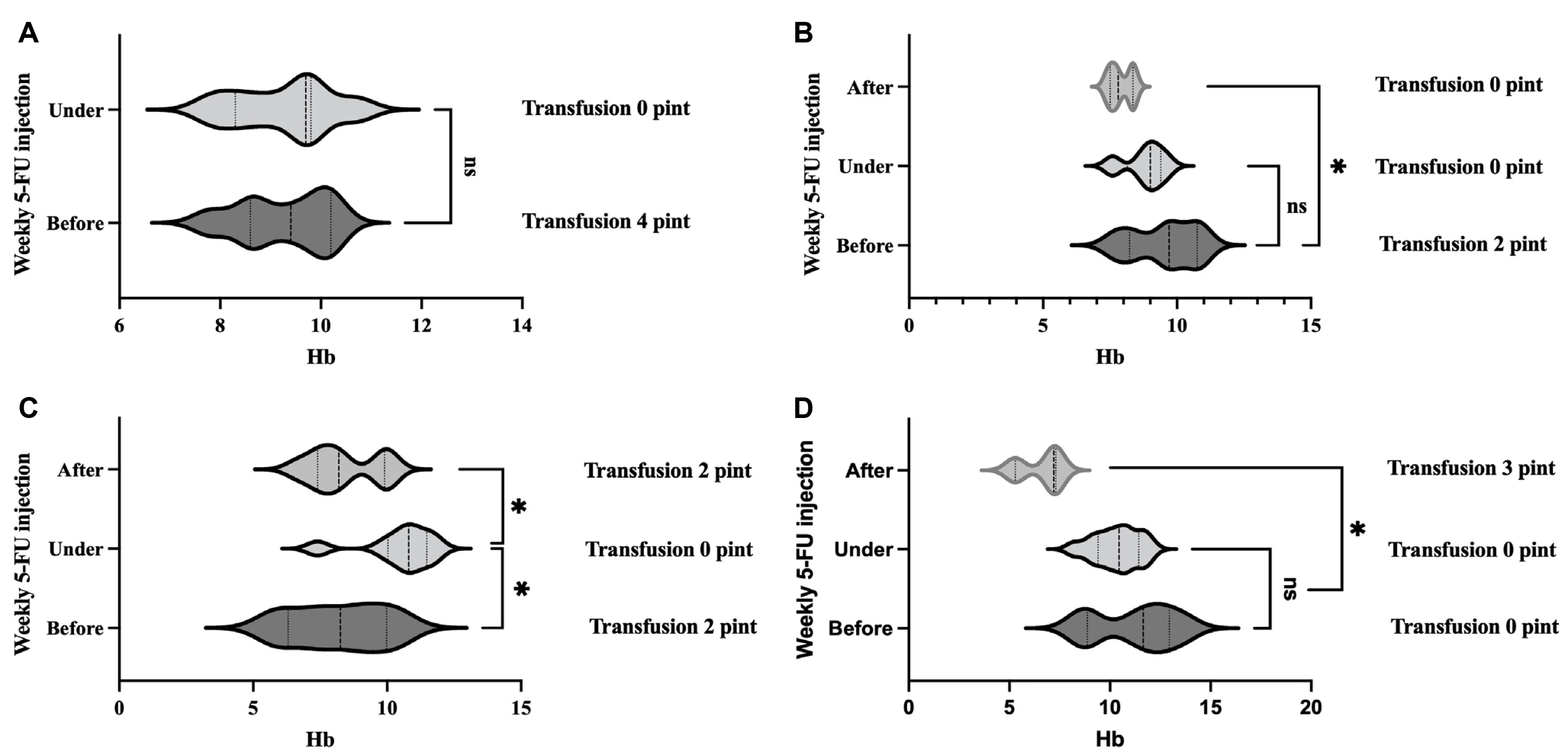
Fig. 4
Comparison of transfusion volume before and during treatment in patients. When transfusion volumes of all patients were compared before and during treatment, a significant decrease was seen after treatment (*p<0.05).
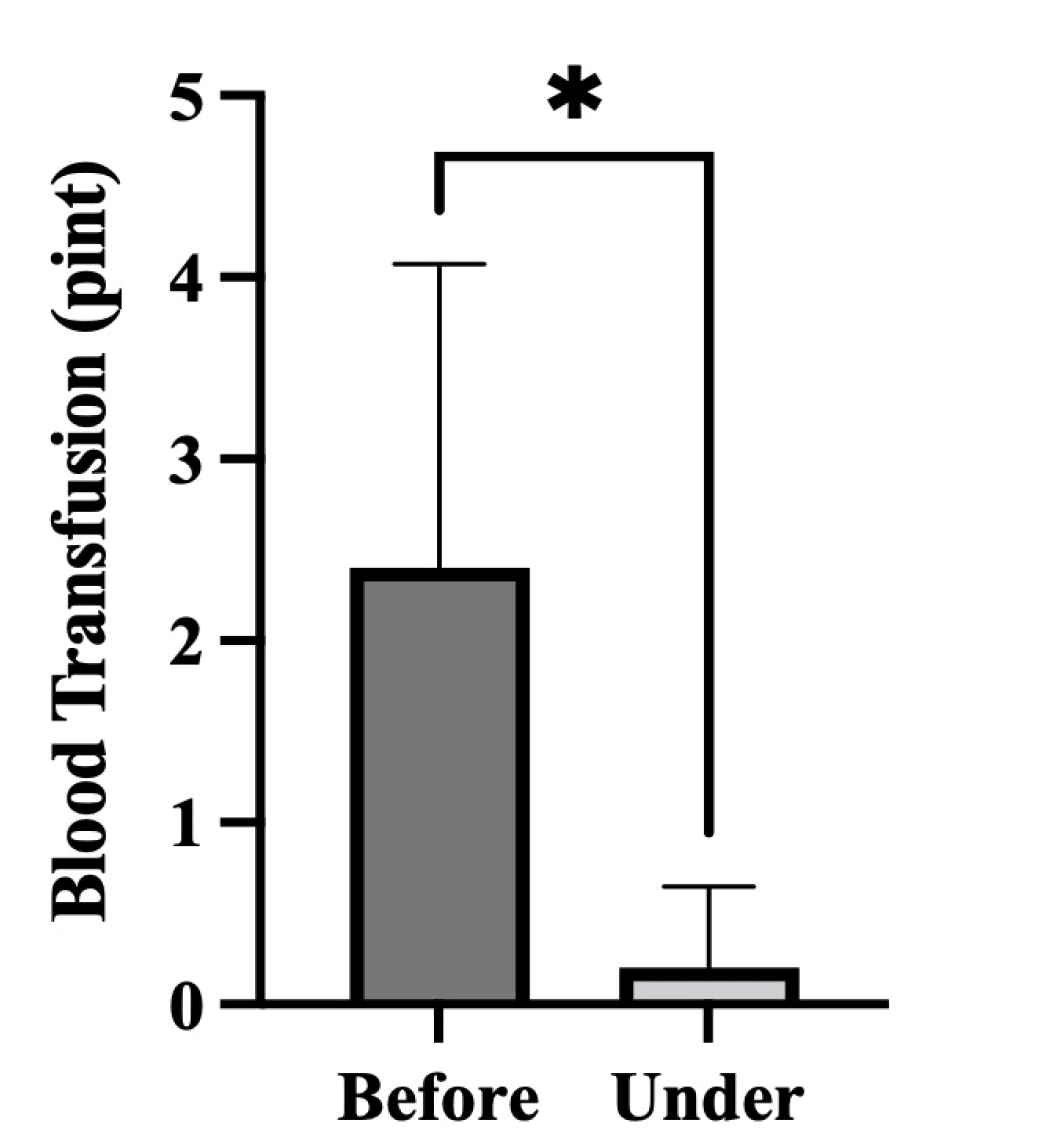
Fig. 5
Immunostaining of thrombospondins 1 (TSP-1) before and after 5-FU injection and expression of TSP-1 induced by 5-FU in human gastric cancer cell line. Expression levels of TSP-1 was increased after injection compared to those before injection (A) and the expression increased significantly in proportion to the concentration of 5-FU (p<0.05, n=5; ×200). *p<0.05, n=5 (B). VEGF, vascular endothelial growth factor.
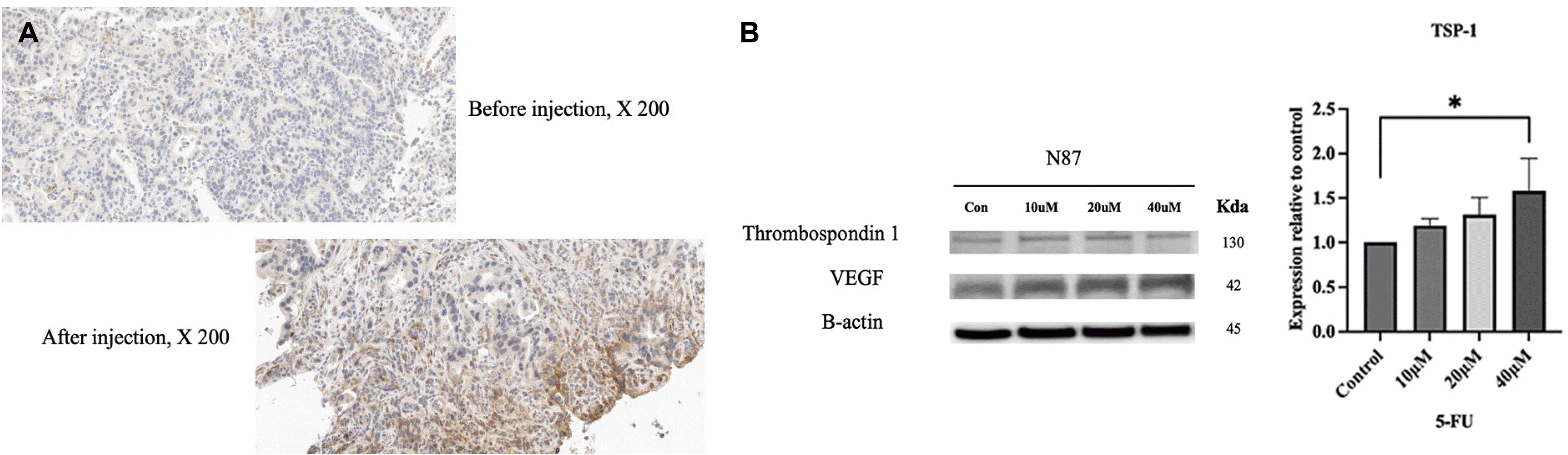
Fig. 6
Changes in blood test results during 5-FU injection treatments. Blood test results during 5-FU injection showed no significant change in SGOT (A)/SGPT (B), total bilirubin (C), or creatinine level (D) (p>0.05). SGOT, serum glutamic oxalacetic transaminase; SGPT, serum glutamic pyruvic transaminase; T.bil, total bilirubin.
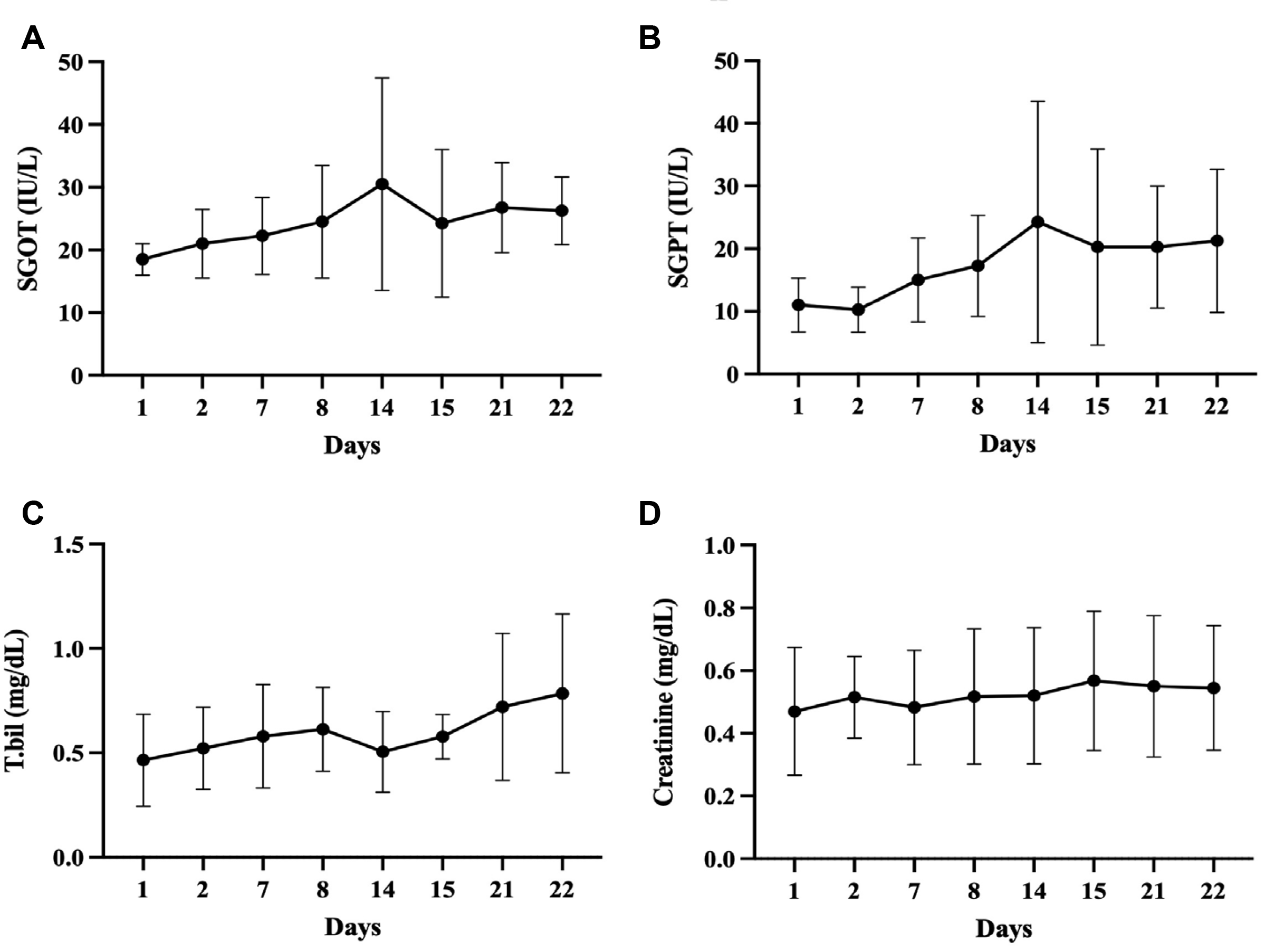
Table 1
Details of Patients Who Received 5-FU Peritumor Injection
| Patients No. | Age (years) | Sex | Size (mm) | Histologic type | Metastatic site | Hemoglobina (g/dL) | Albumina (g/dL) | Injection No. |
|---|---|---|---|---|---|---|---|---|
| 1 | 77 | F | 70 | Tub2 | Liver, spleen, peritoneum | 10.2 | 2.5 | 4 |
| 2 | 69 | F | 40 | Por | Bone, Liver | 9.6 | 3.2 | 4 |
| 3 | 65 | M | 50 | Tub2 | Peritoneum | 7.4 | 2.8 | 8 |
| 4 | 68 | M | 30 | Tub2 | Peritoneum | 9.0 | 2.8 | 12 |
| 5 | 40 | M | 35 | Por | Peritoneum | 8.9 | 2.3 | 10 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download