INTRODUCTION
Acute phlegmonous esophagitis is caused by bacterial infection of the submucosal and muscular layers of the esophagus and may lead to esophageal abscess as a complication. Although it is a rare disease, serious complications such as sepsis, mediastinitis, and esophageal perforation can occur if it is not treated promptly, leading to a high mortality rate.
1 Therefore, if esophageal abscess is suspected, immediate administration of empirical broad-spectrum antibiotics, therapeutic fasting, and abscess drainage is required. Surgical treatment for esophageal abscess was once the standard of care intervention, but with the advancement of endoscopic technology, endoscopic abscess drainage is now the preferred method.
2 Endoscopic drainage has the advantage of being less invasive than surgical treatment, having a shorter recovery time, and being able to avoid complications associated with general anesthesia. However, we should be aware that complications of esophageal stricture may occur due to damage to the esophageal mucosa.
3 In this case, a patient with dysphagia and epigastric pain was diagnosed with an esophageal abscess induced as a complication, secondary to acute phlegmonous esophagitis. After receiving therapeutic fasting, empirical broad spectrum antibiotic infusion, and endoscopic drainage during hospitalization, his symptoms subsided and was discharged from the hospital. Dysphagia recurred 1 month after discharge, and the endoscopic findings revealed a new esophageal stricture. We present a case of esophageal stricture secondary to phlegmonous esophagitis, which improved after active treatment, such as esophageal balloon dilatation, esophageal self-expandable metal stent insertion, and bougienage. We present the case report supported by a thorough literature review.
Go to :

CASE REPORT
A 67-year-old man underwent an endoscopy to find the cause of dysphagia that occurred after eating grilled fish 5 days prior. Endoscopy revealed a defect in the mucosal lining of the esophagus and an esophageal abscess, following which the patient was transferred to our hospital. The patient's vital signs were stable, and he had no comorbidities other than type 2 diabetes. The patient presented with dysphagia and epigastric pain, but no tenderness or swelling in the neck area was observed on physical examination. There was no difference in dysphagic symptoms between liquid and solid food. The blood parameters were as follows: white blood cells 7,750/mm
3 (normal range, 4,000-11,000/mm
3), hemoglobin 14.0 g/dL (normal range, 13.5-17.5), blood urea nitrogen 9.2 mg/dL (normal range, 6.6-23.6), creatinine 0.8 mg/dL (normal range, 0.67-1.17), C-reactive protein 2.05 mg/dL (normal range, 0-0.5), fasting glucose 120 mg/dL (normal range, 74-106), and no bacteria were observed in blood culture tests. Chest CT disclosed diffuse thickening of the esophageal wall and an abscess at the gastroesophageal junction (
Fig. 1). Gastric endoscopy showed that an abscess of approximately from 23 cm to 45 cm of the incisor teeth (IT) accompanied with 2 cm sized mucosal defect on IT 23 cm of esophageal wall (
Fig. 2). Combining clinical features and laboratory findings, esophageal abscess was diagnosed as a complication of acute phlegmonous esophagitis, following which, empirical antibiotics (piperacillin/tazobactam 4.5 g every 8 hours, ciprofloxacin 400 mg every 12 hours) were administered intravenously while maintaining therapeutic fasting. Endoscopic drainage of the esophageal abscess was planned since his vital signs were stable. Considering gravity, an esophageal mucosal incision was planned from the IT, 40 cm to the gastroesophageal junction. However, the patient's cooperation was poor, and an esophageal mucosal incision was made from the IT 23 cm to 40 cm (
Fig. 3). Nevertheless, on a follow up CT of the neck performed one week later, the abscess was still found in the lower esophagus; therefore, an additional incision was made from the IT 40 to 45 cm, and therapeutic fasting and intravenous antibiotic treatment were continued. Esophagography was performed on the 14th day of hospitalization to check micro-perforation of the esophagus and esophageal stricture caused by frequent endoscopic procedures. There were no signs of contrast leakage and esophageal stricture, so the porridge diet was started. The patient did not report any discomfort while eating. The patient's symptoms subsided and he was discharged on the 20th day of admission and oral antibiotics (levofloxacin 500 mg everyday) were taken for another 3 weeks.
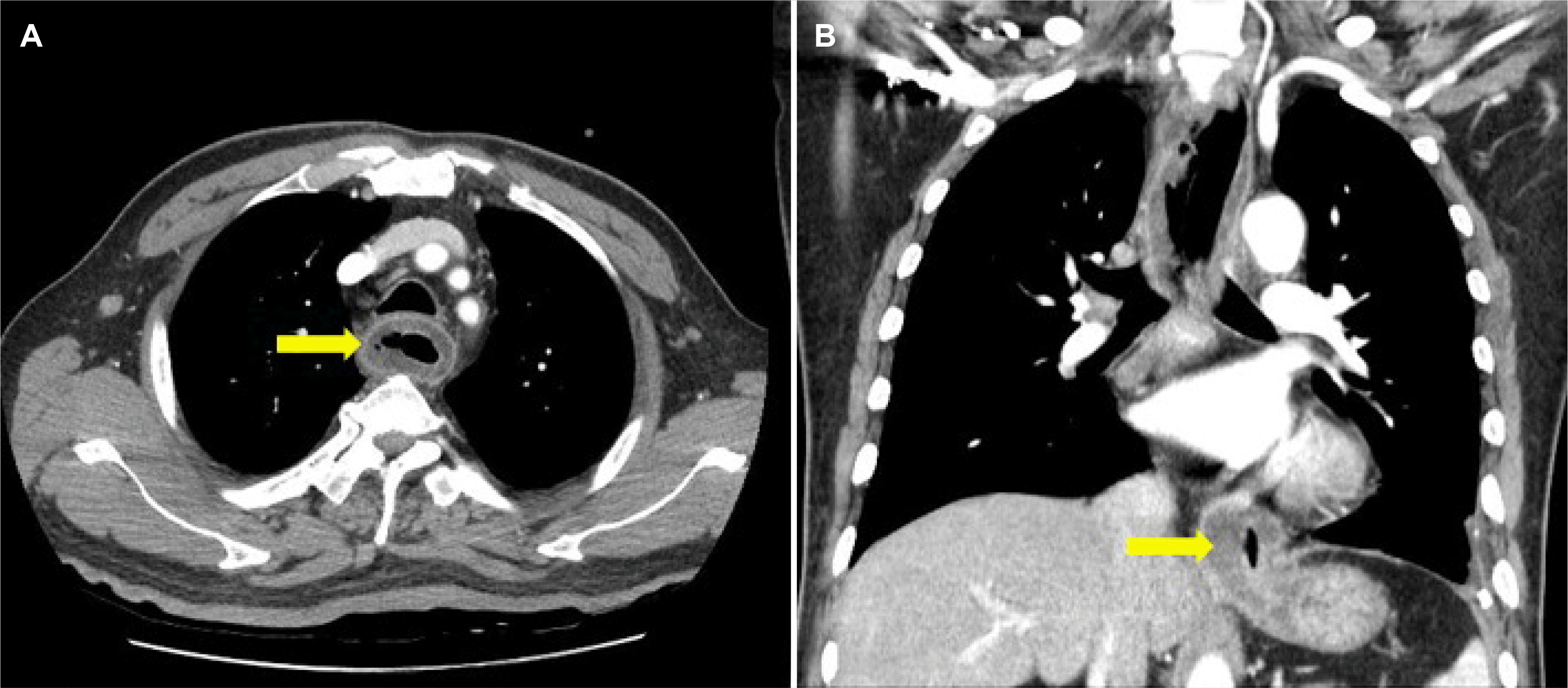 | Fig. 1Initial chest CT image. (A) Diffuse wall thickening of the esophageal wall and air-bubble sign (arrow) were observed. (B) Esophageal dilatation due to the mass like lesion (arrow) of the gastroesophageal junction is observed. 
|
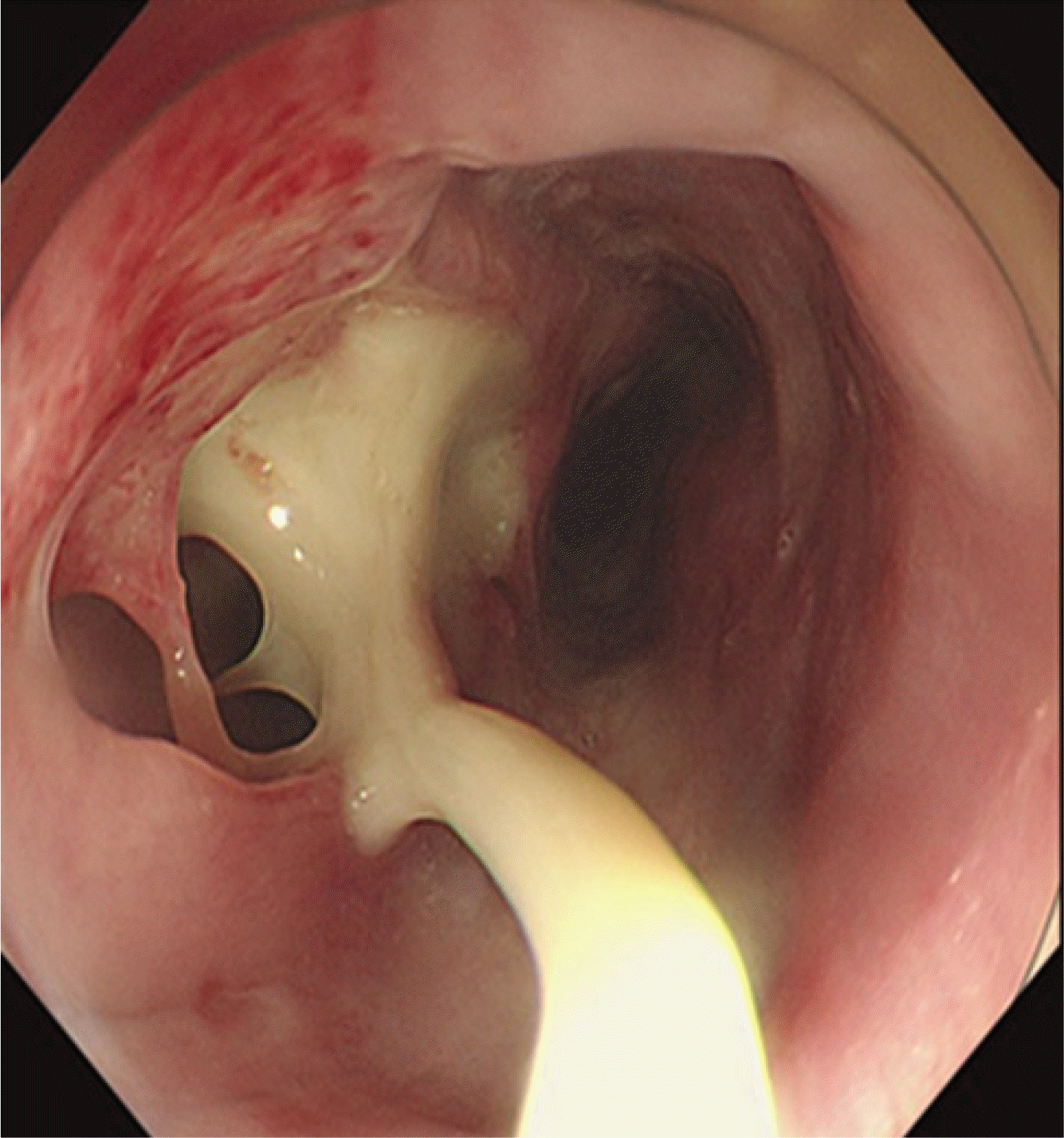 | Fig. 2Initial gastric endoscopy image. An abscess of from 23 cm to 45 cm of the incisor teeth accompanied with 2 cm sized mucosal defect on incisor teeth 23 cm of esophageal wall. 
|
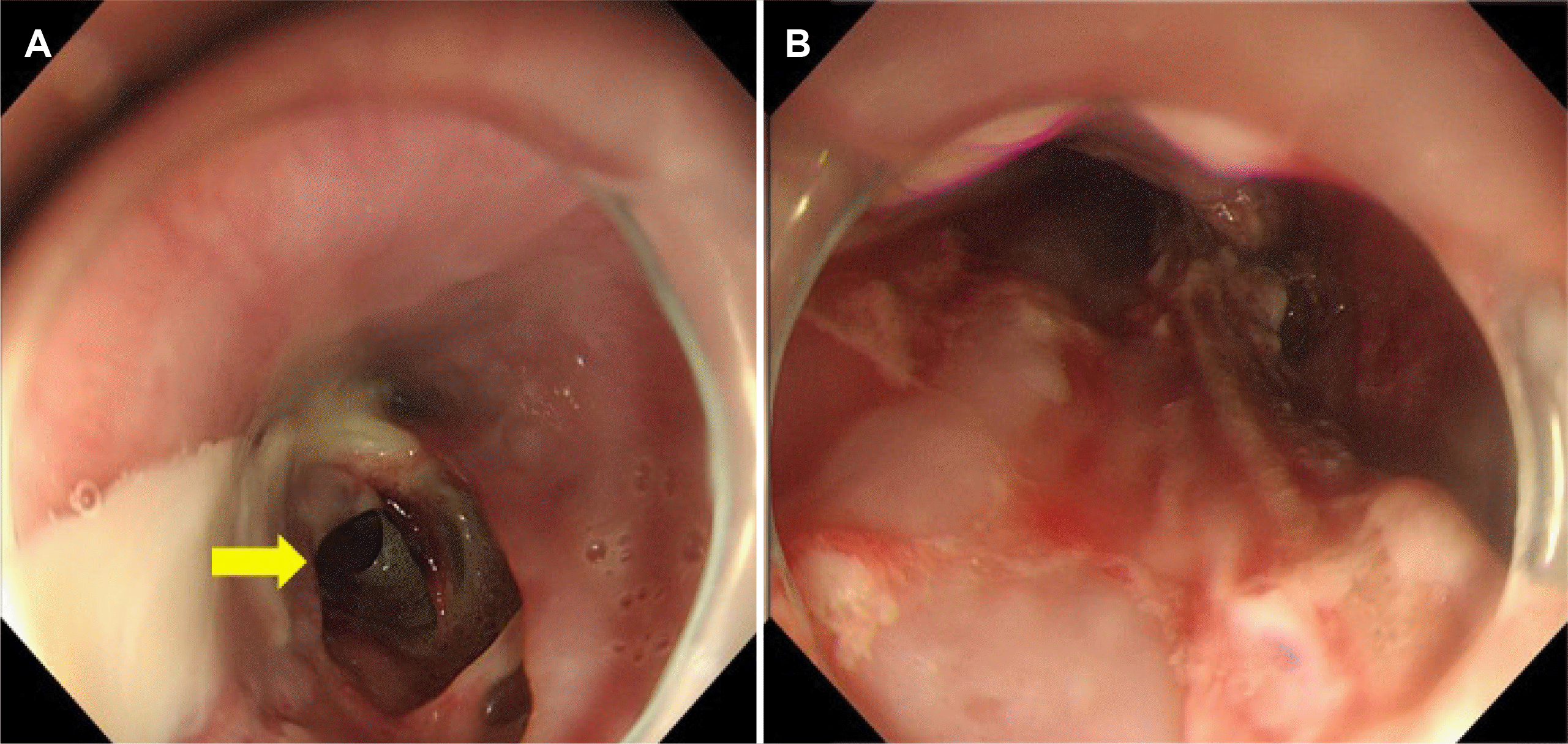 | Fig. 3Endoscopic drainage of an abscess in the esophagus. (A) Abscess lumen (arrow) is observed. (B) Esophageal mucosal incision was made from the incisor teeth 23 cm to 40 cm. 
|
However, the dysphagia recurred 1 month after discharge, an esophageal stricture was newly revealed at IT 25 cm, and the endoscope was not passed due to stricture (
Fig. 4). We confirmed esophageal stricture from IT 25 cm to IT 40 cm by using small-caliber endoscope. To improve the symptoms of dysphagia and prevent recurrence of esophageal stricture, endoscopic balloon (Controlled radial expansion balloon, Boston Scientific, Marlborough, MA, USA) dilatation was performed twice for 1 minute at 2-week intervals (8-9-10 mm, 10-11-12 mm, 12-13.5-15 mm), but there was no improvement in dysphagia or esophageal stricture (
Fig. 5). Therefore, a self-expandable covered 12 cm metal stent (Hanarostent; M.I. Tech Co, Seoul, Korea) was inserted into the lower part of the esophagus (
Fig. 5). The patient's symptoms improved 3 weeks after insertion of the esophageal stent, and esophageal stent removal was performed. Oral steroids (prednisolone 30 mg/day for 2 weeks, then reduced by 5 mg weekly) were simultaneously administered to prevent restricture. Nevertheless, the improvement of the esophageal stricture was slow, and dysphagia persisted. Therefore, 8 additional bougienage (Savary-Gilliard dilators; Cook Medical, Winston-Salem, NC, USA) procedures (from 28 French to 56 French) were performed in collaboration with the thoracic surgeon at 2 weeks intervals, and the symptoms and esophageal stricture improved (
Fig. 5). The patient has not complained of dysphagia or any abnormal symptoms so far and is being followed up at the outpatient clinic.
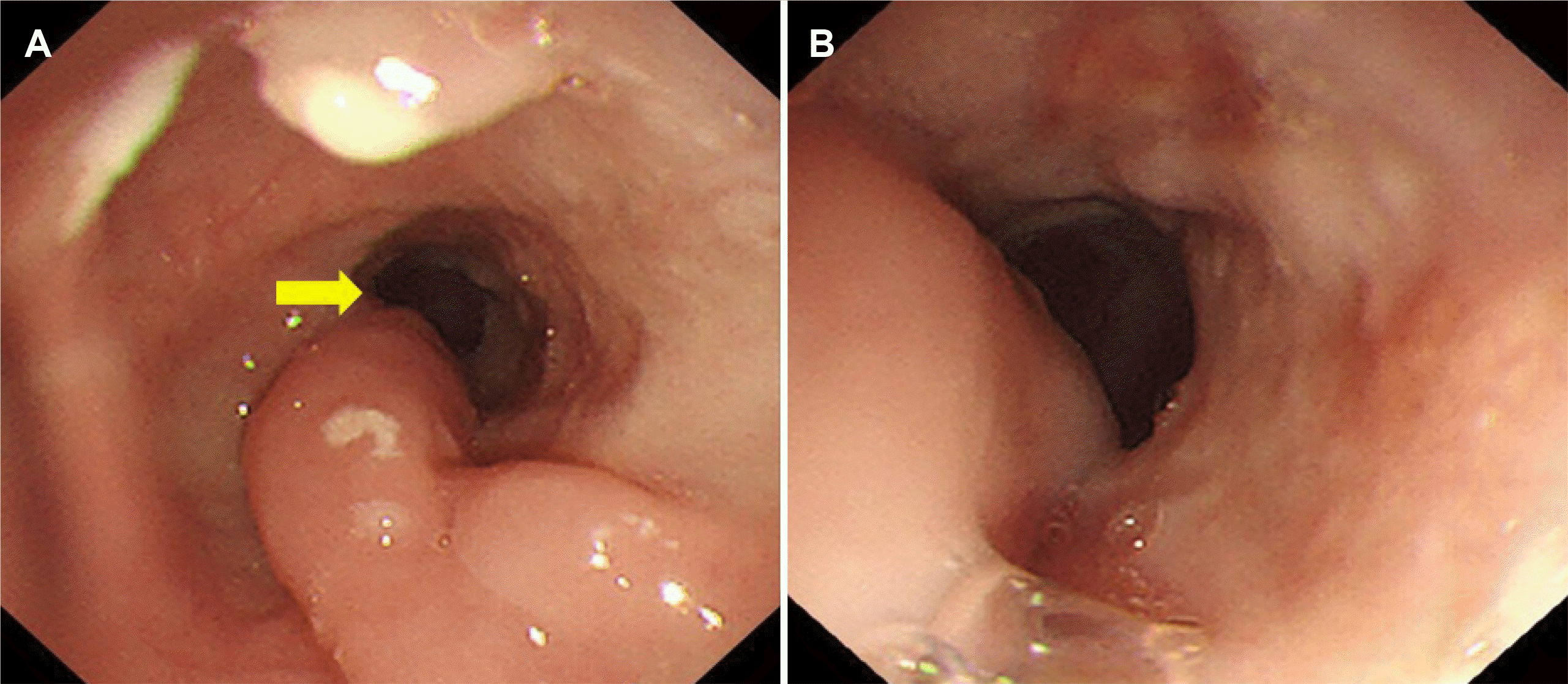 | Fig. 4Esophageal stricture (arrow) was newly revealed following endoscopy. 
|
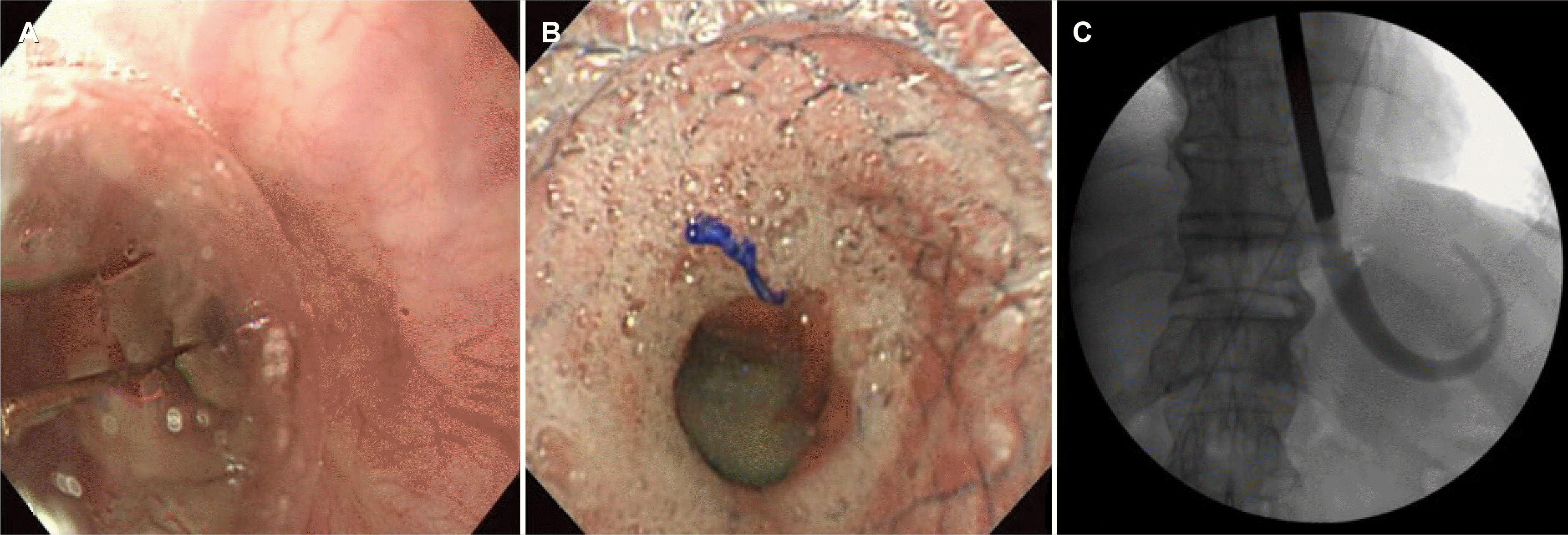 | Fig. 5Treatment for esophageal stricture. (A) Endoscopic balloon dilation. (B) Esophageal self-expandable metal stent insertion. (C) Esophageal lumen dilatation using the bougienage. 
|
Go to :

DISCUSSION
Esophageal abscess is a serious disease with high mortality, and rapid drainage is essential.
4 Endoscopic abscess drainage through esophageal mucosal incision is less invasive than surgical surgery and can reduce postoperative complications that can occur due to general anesthesia. Therefore, it has recently been spotlighted as an effective treatment for patients who cannot tolerate general anesthesia or who are concerned about complications after surgery.
3,5 However, extensive mucosal damage to the esophagus induced by endoscopic drainage can stimulate the overproduction of fibrous tissue, leading to complications such as esophageal stricture.
6 Esophageal stricture is an abnormal narrowing of the esophagus lumen and is a disease with a low prevalence of 1.1 per 10,000 person-years, but with significant adverse effects in affected patients.
7 It is known that 70-80% of esophageal stricture cases are caused by long-term gastroesophageal reflux disease and may occur even after erosive stenosis, surgical anastomosis stenosis, and endoscopic submucosal dissection.
8 It is known that 70-80% of esophageal stricture cases are caused by long-term gastroesophageal reflux disease and may occur even after erosive stenosis, surgical anastomosis stenosis, and endoscopic submucosal dissection.
8 In the case of endoscopic submucosal dissection procedure, it is known that about 70-90% of esophageal stricture can be caused as a complication, and it is related to the size of the lesion and the extent of the surrounding mucosal defect.
9 However, esophageal stricture as a complication of endoscopic abscess drainage has not been reported before, so it shows a characteristic that sets it apart from other cases.
Symptoms associated with esophageal stricture include dysphagia, pain, malnutrition, and aspiration.
7 When esophageal stricture occurs, it is difficult to expect an improvement of symptoms with conservative treatment alone, so active interventional treatment is required.
10 Treatment options include endoscopic balloon dilatation, esophageal lumen dilatation using the bougienage, esophageal stent insertion, and surgical resection.
11 Recently, with the development of therapeutic endoscopy technology, endoscopic esophageal dilatation is being actively performed. Endoscopic balloon dilatation or bougienage is an effective and safe treatment because of its relatively simple procedure and small size; however, the effect of endoscopic dilatation is limited when the length of the stenosis is long.
11 Esophageal stents are often used in patients with refractory stricture, and by using a stent to keep the esophageal lumen open for a long time, the surrounding tissue is remodeled, and the stenosis does not recur even after stent removal. Although several studies have demonstrated the validity of stenting for refractory esophageal anastomosis stenosis, complications such as stent movement, pain, and tissue overgrowth have been observed, and further studies on long-term efficacy are needed.
12 Steroid injections into the stricture site or oral steroid administration are used as adjuvant treatments. Steroids are supposed to reduce the likelihood of recurrence of the stricture, as they help reduce inflammation and damage from swelling by inhibiting the inflammatory response and reducing collagen formation.
13 Several retrospective studies have shown that the combination of steroid injection and balloon dilatation can reduce the total number of dilatations required for esophageal anastomosis stenosis, but long-term data are needed due to controversy over the safety and efficacy.
14 Esophageal stricture is a disease that emphasizes the importance of prevention because the incidence rate is high at 10-30% regardless of the cause and treatment method. Currently, many approaches for stricture prevention, such as the use of anti-inflammatory drugs, have been applied to these strictures.
15 But there is no standard preventive treatment recommended so far since its efficacy is insufficient, and it has not been implemented in clinical practice.
15
In conclusion, endoscopic abscess drainage for the treatment of esophageal abscess is a less invasive and efficient procedure, but it should be kept in mind that complications such as esophageal stricture may occur because of damage to the esophageal mucosa. To reduce the risk of complications, we propose to limit the scope of esophageal mucosal resection through an incision at the bottom of the abscess in consideration of gravity. We also report that a step-by-step treatment strategy of performing mucosal incisions at timed intervals is necessary to prevent esophageal stricture.
Go to :






 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download