This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
This study determined the effect of platelet-rich fibrin (PRF) on extraction socket bone regeneration and assessed the patterns and determinants of bone regeneration after the surgical extraction of impacted mandibular third molars.
Materials and Methods
This prospective study randomly allocated 90 patients into two treatment groups A PRF group (intervention group) and a non-PRF group (control group). After surgical extractions, the PRF group had PRF placed in the extraction socket and the socket was sutured, while the socket was only sutured in the non-PRF group. At postoperative weeks 1, 4, 8, and 12, periapical radiographs were obtained and HLImage software was used to determine the region of newly formed bone (RNFB) and the pattern of bone formation. The determinants of bone regeneration were assessed. Statistical significance was set at P<0.05.
Results
The percentage RNFB (RNFB%) was not significantly higher in the PRF group when compared with the non-PRF group at postoperative weeks 1, 4, 8, and 12 (P=0.188, 0.155, 0.132, and 0.219, respectively). Within the non-PRF group, the middle third consistently exhibited the highest bone formation while the least amount of bone formation was consistently observed in the cervical third. In the PRF group, the middle third had the highest bone formation, while bone formation at the apical third was smaller compared to the cervical third at the 8th week with this difference widening at the 12th week. The sex of the patient, type of impaction, and duration of surgery was significantly associated with percentage bone formation (P=0.041, 0.043, and 0.018, respectively).
Conclusion
Placement of PRF in extraction sockets increased socket bone regeneration. However, this finding was not statistically significant. The patient’s sex, type of impaction, and duration of surgery significantly influenced the percentage of bone formation.
Go to :

Keywords: Platelet-rich fibrin, Bone regeneration, Third molar
I. Introduction
Extraction socket bone regeneration represents a special type of bone healing as it usually occurs in the oral environment which is continually bathed with saliva containing numerous micro-organisms
1. This healing also takes place with the presence of remnants of periodontal ligament fibers within the socket walls and thus presents a scenario that is different from bone healing in other skeletal sites
2. Bone removal is often necessary for the surgical removal of impacted mandibular third molars (M3)
3.
An impacted tooth is a tooth that is prevented from erupting due to a physical barrier within the path of eruption, malposition, or lack of space in the arch
4. M3 are the most frequently impacted teeth and may be prevented from erupting into the oral cavity through hard or soft tissue impactions
5. They may be partially or completely covered by bone which must be removed before tooth extraction. There are various methods of bone removal during mandibular third molar surgery including the more popular disto-buccal guttering technique which requires the removal of bone buccal and distal to the impacted tooth to facilitate tooth elevation and extraction. The amount of bone removed during these procedures varies and are largely dependent of the depth of impaction and angulation of the impacted tooth within the mandible. Although some level of spontaneous bone regeneration occurs in the extraction sockets after M3 surgery, some M3 procedures may be associated with the periodontal compromise of the mandibular second molar as a result of a residual alveolar defect on the distal aspect of the mandibular second molar, loss of attachment, and periodontal pocket formation
6. During M3 surgery, it is desirable to optimize bone regeneration to minimize the periodontal compromise of the mandibular second molar.
Platelet-rich fibrin (PRF) is an autologous second generation platelet concentrate discovered by Choukroun et al.
7 in 2001. It is a biomaterial that exhibits promising prospects in enhancing bone regeneration in extraction sockets. PRF concentrates platelets rich in growth factors and cytokines into a fibrin clot
8. Growth factors released by these platelets have the potential to promote bone healing by enhancing the mitogenesis, migration, and matrix synthesis of osteoblasts
9. PRF releases growth factors vital to wound healing including platelet derived growth factor (PDGF), transforming growth factor β (TGF-β), IGF (insulin-like growth factor), EGF (epidermal growth factor), VEGF (vascular endothelial growth factor), fibrinogen, factor V, factor VII, angioproteins, and platelet factor 4
10,11. One of the highest concentrations of PDGF and TGF-β in the body is found within platelets. PDGF and TGF-β contribute to bone regeneration and increased vascularity. The alpha granules of platelets fuse with its cell membrane upon activation. Secretory proteins contained in the granules are transformed to a bioactive state and secreted. These secretory proteins then bind to their target cells causing intracellular signal proteins to be activated. This results in the expression of a gene sequence that directs cellular proliferation, collagen synthesis, and osteoid production
11. PRF has the advantage of being autologous and presents an affordable and safe alternative for enhancing bone regeneration
12,13.
In recent literature, attempts have been made to study the effect of PRF on bone regeneration in extraction sockets. However, reports from these studies are contradictory and inconclusive. Some studies have reported that bone regeneration in the PRF group was not significantly better compared to the controls
14-17. Other studies have reported that PRF significantly improved bone regeneration in extraction sockets compared to the controls
1,18-20. The variation in reported outcomes may be attributed to the limited sample sizes used in most studies which ultimately would have affected the power of the study. Variations in the PRF preparation protocol used may also account for the heterogeneity in the results. Most studies in the literature did not report on the pattern of bone formation within the extraction sockets grafted with PRF, and only reported the average percentage density of newly formed bone within the extraction socket. In assessing the ability of PRF to improve bone regeneration, it is important to determine the region of the extraction socket (cervical, middle, or apical third) stimulated to form new bone by PRF. Previous studies have reported that age
21,22 and sex
23 may affect bone healing. Other studies have reported that age, sex, volume of local anesthetic used, impaction type, and surgery duration can influence the inflammatory process associated with healing
24,25. However, no study has investigated the pattern of bone formation produced by PRF or the effects of age, sex, type of impaction, amount of local anesthesia used, duration of surgery, and associated pathology on the bone regenerative potential of PRF. The attendant limitations of this approach make the results of most previous studies heterogeneous with a consequent need for further studies.
Therefore, this study determined the effect of PRF on extraction socket bone regeneration, the pattern of bone regeneration, and the determinants of socket bone regeneration after the surgical extractions of impacted mandibular third molars.
Go to :

II. Materials and Methods
This study was approved by the ethical research committee of Obafemi Awolowo University Teaching Hospital Complex (OAUTHC), Ile-Ife, Nigeria (No. IRB/IEC/0004553). This study also adhered to the Declaration of Helsinki on medical protocol and ethics, 2013, and written informed consent was obtained from all patients.
A pilot study composed of 10 patients with impacted M3s indicated for surgical extraction was randomized into PRF and non-PRF groups. This was used to determine the reliability of HLImage software, the measuring instrument for bone density. A split-half reliability test was performed and yielded a Spearman–Brown’s coefficient of 0.93, indicating a high reliability. The pilot study also assisted in the calibration of the surgeon and two research assistants, thus ensuring reliable data collection. The data obtained from the pilot study was excluded from the data analysis of this study.
This prospective clinical study included 90 patients aged 18-35 years presenting with a diagnosis of impacted M3s that were indicated for surgical extractions under local anesthesia between October 2017 and June 2018 at the Oral and Maxillofacial Surgery Clinic of OAUTHC and agreed to 3 months of follow-up visits. Patients with systemic diseases, using systemic drugs, on anti-platelet or anticoagulant therapy, allergic to penicillin, possessing acute local infections, smokers, using oral contraceptives, are pregnant or lactating, or have a history of jaw irradiation were excluded.
Each patient participated as a volunteer after signing an informed consent form. Ninety patients were randomized into two groups—PRF and non-PRF groups using an online generated sequence from GraphPad Software (
https://www.graphpad.com/). Preoperatively, patients’ demographic and clinical variables such as FDI (Fédération dentaire internationale) notation, type of impaction (Winter’s classification), Pederson’s difficulty index, and associated pathology of the tooth to be extracted were charted. The baseline platelet count for those in the PRF group was determined.
The included participants underwent surgical extractions of impacted mandibular third molars under local anesthesia. All the surgical extractions were performed by one surgeon under the same standardized protocol of the buccal guttering technique. PRF was prepared (
Fig. 1) and placed in the extraction socket of the participants in the PRF group (
Fig. 2), while blood clot was allowed to fill the extraction sockets of the participants in the non-PRF group. The sockets were subsequently sutured using 3/0 black silk sutures. Suture removal was performed one week postoperatively at the first follow-up visit. Intraoperatively, records taken included the amount of local anesthetic used in milliliters and the duration of the surgery in minutes. Verbal and written postoperative instructions were provided to the patients. All patients received the same antibiotics (amoxicillin [Beecham Amoxil capsule 500 mg; GSK, Brentford, UK] and metronidazole 400 mg every 8 hours for 5 days) and analgesics (ibuprofen capsule 400 mg every 8 hours for 3 days). The patients were instructed not to take any other medications.
 | Fig. 1Platelet-rich fibrin prepared after the centrifugation of 10 mL of autologous whole blood at 3,000 rpm for 10 minutes. 
|
 | Fig. 2Platelet-rich fibrin (PRF) inserted into the extraction socket of a participant in the PRF group. 
|
1. Platelet-rich fibrin preparation protocol
PRF was prepared according to the technique described by Dohan et al.
26. Fifteen minutes prior to surgery, 10 mL of venous whole blood was collected (from the patients in the PRF group) in a 10 mL glass-coated plastic vacutainer tube (Axiom vacuum sterile tube) without anticoagulants. The specimen was centrifuged immediately (Axiom centrifuge, Model 800B; Axiom Medical, London, UK) at 3,000 rpm for 10 minutes.
The centrifuged blood was obtained in three fractions: an upper straw colored layer containing acellular plasma (platelet poor plasma fraction), the middle layer containing the fibrin clot (PRF fraction), and a red lower layer containing red blood cells (red cell fraction).
The PRF obtained was placed in the extraction sockets of the participants in the PRF group and the sockets were sutured.
2. Radiographic evaluation
Radiographic bone healing was assessed by determining the percentage density of newly formed bone in the extraction sockets using HLImage software according to Célio-Mariano et al.
27.
Standardized periapical radiographs (Primax RDX-58E soft; Primax, Berlin, Germany) were taken at postoperative weeks 1, 4, 8, and 12 using a Rinn’s (XCP; Dentsply, Charlotte, NC, USA) film holder with the paralleling technique. The films were automatically processed (Periomat Plus; Dürr Dental, Bietigheim-Bissingen, Germany). Radiographic images were digitized with a fixed camera (SONY Cybershot DSC-HX200V, 18 megapixels; SONY, Tokyo, Japan). Digitization involved mounting the processed periapical radiographs on a viewing box (HUION LED Light Pad, L4S; HUION, ShenZhen, China) and the camera with fixed settings was positioned 2 cm horizontally away from the radiograph with its lens perpendicular to the radiograph. The standardized digitized images were subsequently transferred to HLImage++ software (ver. PCM: 18.0.3.q; Western Vision Software, Layton, UT, USA) (
Fig. 3) for the analysis of bone healing.
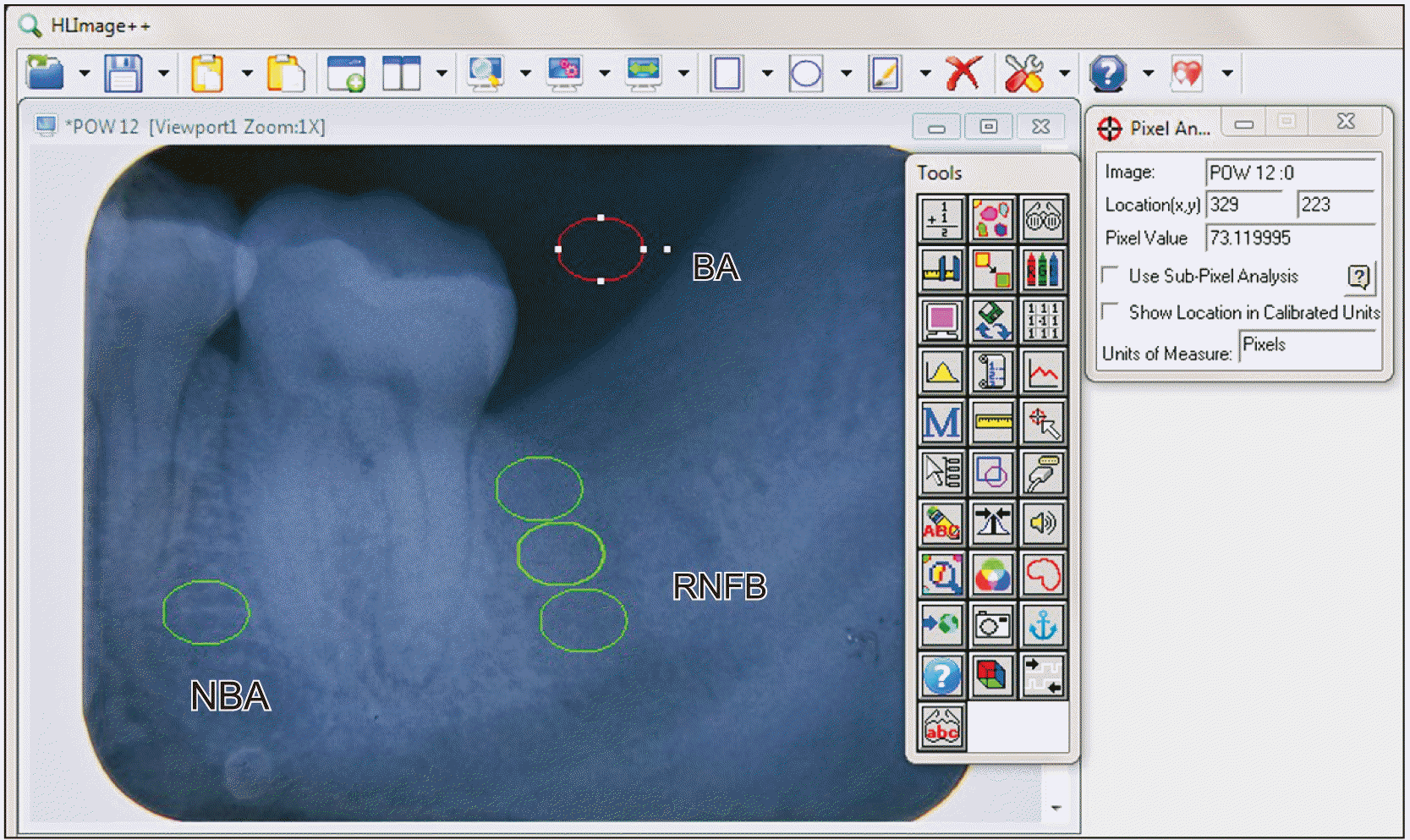 | Fig. 3Assessment of radiographic density of newly formed bone in the extraction socket using the HLImage++ software (ver. PCM: 18.0.3.q; Western Vision Software). The background area (BA), natural bone area (NBA), and the region of newly formed bone (RNFB) in the cervical, middle and apical thirds of the extraction socket are shown. 
|
Five areas were defined on the radiographic image for quantification of the density of new bone formed in the patients. The five areas were: (1) cervical, (2) middle, (3) apical third of the extraction sockets corresponding to the region of newly formed bone (RNFB), (4) the natural bone area (NBA) to act as the standard comparing to RNFB, and (5) the background area (BA) to minimize any error caused by the contrast of the radiographic image.(
Fig. 3) Measurements were taken in pixels (256 grayscale). The NBA was defined as the interdental bone area between the first and second mandibular molars on the ipsilateral side, while BA is the background area superior to the extraction socket of the mandibular third molar. Placement of the RNFB was standardized to be below the external oblique line of the mandible to the apical third of the extraction socket. The radiographic density of each socket was calculated as the mean of the radiographic density of all thirds (cervical, middle, and apical) of each extraction socket. The quantification of the percentage density of the RNFB (RNFB%) equals
27:
Radiographic evaluation was performed by a blinded radiologist in the Department of Radiology, Obafemi Awolowo University.
3. Data analysis
Data analysis was carried out using IBM SPSS (ver. 20.0; IBM, Armonk, NY, USA) and Stata (ver. 16; StataCorp LLC, College Station, TX, USA). Descriptive statistics were obtained for socio-demographic variables such as age, sex, marital status, and occupation. The means of percentage region of newly formed bone (RNFB%) was determined and compared between the groups. The independent t -test was used to test the statistical significance of the continuous variables for bone healing. We conducted a one-way multivariate ANOVA (MANOVA) of percentage bone formation at the cervical, middle, and apical portions of the tooth for postoperative follow-up measurements at weeks 1, 4, 8, and 12 separately for the intervention and control groups. We used these findings to visualize the linear prediction of percentage bone formation in the extraction sockets by plotting the predicted margins following model specification. Subsequently, we conducted a two-way MANOVA of percentage bone formation at the cervical, middle, and apical portions of the tooth across postoperative follow-up measurements at weeks 1, 4, 8, and 12 with the study group membership. Finally, we conducted a mixed-effect regression modelling of percentage bone formation in the cervical, middle, and apical portions of the tooth with a robust estimation of variance, comparing the intervention and control groups, and controlling for the timing of follow-ups and other demographic and clinical characteristics of the patients. Patients’ unique identifiers were used as random effect parameters.
The data was presented using tables and graphs. Statistical significance was inferred at P<0.05 and based on a 95% confidence interval.
Go to :

III. Results
There were 90 participants with a total of 6 drop-outs and an attrition rate of 6.67%.(
Fig. 4) The mean age of the participants in the PRF group was 26.49±4.84 years and 25.09±4.20 years in the non-PRF group. The age range of the participants in both groups was 18-35 years. The two groups did not differ significantly in age, sex (
P=0.407 and
P=0.280, respectively), indication for extraction, impaction type, Pederson’s difficulty index, and associated pathology.(
Table 1) The platelet count of the participants in the PRF group ranged from 157,000 to 400,000 cell/µL. The mean platelet count was 278,177.78±51,206.93 cells/µL.
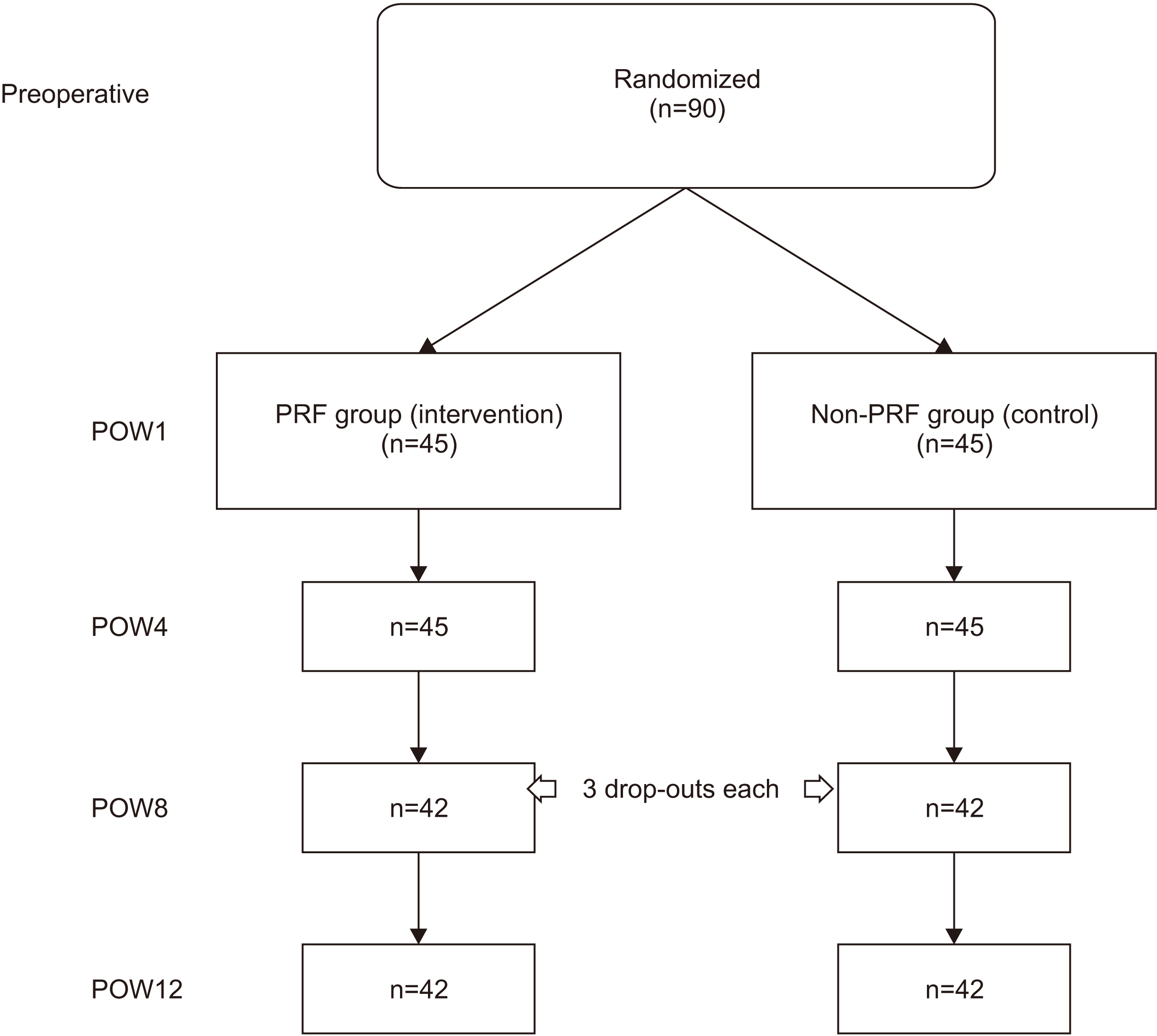 | Fig. 4Flow chart of participants in the platelet-rich fibrin (PRF) and non-PRF groups. (POW: postoperative week) 
|
Table 1
Preoperative tooth assessment of the participants in the PRF and non-PRF groups
|
Variable |
PRF group (n=45) |
Non-PRF group (n=45) |
Total (n=90) |
χ2
|
df |
P-value |
|
Indication for extraction |
|
|
|
2.295 |
3 |
0.514 |
|
Pericoronitis |
31 (68.9) |
34 (75.6) |
65 (72.2) |
|
|
|
|
Apical periodontitis |
12 (26.7) |
9 (20.0) |
21 (23.3) |
|
|
|
|
Irreversible pulpitis |
1 (2.2) |
2 (4.4) |
3 (3.3) |
|
|
|
|
Orthodontic reasons |
1 (2.2) |
0 (0.0) |
1 (1.1) |
|
|
|
|
Impaction type (Winter’s classification) |
|
|
|
3.008 |
3 |
0.390 |
|
Mesioangular |
17 (37.8) |
10 (22.2) |
27 (30.0) |
|
|
|
|
Horizontal |
9 (20.0) |
14 (31.1) |
23 (25.6) |
|
|
|
|
Vertical |
12 (26.7) |
13 (28.9) |
25 (27.8) |
|
|
|
|
Distoangular |
7 (15.6) |
8 (17.8) |
15 (16.7) |
|
|
|
|
Pederson difficulty index |
|
|
|
5.521 |
2 |
0.063 |
|
Easy |
7 (15.6) |
1 (2.2) |
8 (8.9) |
|
|
|
|
Moderately difficult |
29 (64.4) |
33 (73.3) |
62 (68.9) |
|
|
|
|
Difficult |
9 (20.0) |
11 (24.4) |
20 (22.2) |
|
|
|
|
Associated pathology |
|
|
|
1.220 |
2 |
0.543 |
|
No pathology |
5 (11.1) |
4 (8.9) |
9 (10.0) |
|
|
|
|
Periodontal pocket |
26 (57.8) |
31 (68.9) |
57 (63.3) |
|
|
|
|
Periodontal pocket+caries |
14 (31.1) |
10 (22.2) |
24 (26.7) |
|
|
|

Table 2 shows the descriptive statistics of intraoperative variables for the study participants. The amount of local anesthesia used for the participants in the PRF group ranged from 3.6 to 5.4 mL. The mean amount was 3.80±0.57 mL. For the non-PRF group, the amount of local anesthesia used ranged from 3.6 to 5.4 mL with a mean of 4.00±0.76 mL. The difference in the mean quantity of local anesthesia used for the two groups was not statistically significant (
P=0.161).
Table 2
Descriptive statistics of the intraoperative variables
|
Variable |
PRF group (n=45) |
Non-PRF group (n=45) |
P-value |
|
Mean amount of local anesthetic used (mL) |
3.80±0.57 |
4.00±0.76 |
0.161 |
|
Mean duration of surgery (min) |
31.84±9.04 |
26.51±6.94 |
0.002* |
|
Tooth delivery method |
|
|
|
|
Ostectomy+either elevation/forceps |
35 (77.8) |
30 (66.7) |
0.388 |
|
Ostectomy+coronal section |
8 (17.8) |
10 (22.2) |
|
|
Complex extraction (root resection) |
2 (4.4) |
5 (11.1) |
|

The duration of surgery for the PRF group ranged from 17 to 56 minutes and 17 to 42 minutes for the non-PRF group. The mean duration of surgery was statistically significantly longer in the PRF group compared to the non-PRF group (31.84±9.04 minutes and 26.51±6.94 minutes, respectively; P=0.002).
The most common tooth delivery method in both groups was through the use of an ostectomy with either elevation or forceps for 35 participants (77.8%) in the PRF group and for 30 participants (66.7%) in the non-PRF group. Complex extractions (root resection) were the least used tooth delivery method in both groups for two (4.4%) participants in the PRF group and five (11.1%) in the non-PRF group. The difference in the type of tooth delivery method used for the two groups was not statistically significant (P=0.388).
Fig. 5 shows a positive linear trend of bone formation at the cervical, middle, and apical portions of the tooth from weeks 1 to 12 for both the control (non-PRF) and intervention groups (PRF). Within the control group, the middle third consistently exhibited the highest bone formation followed by the apical third, while the least bone formation was consistently observed in the cervical third. For the intervention group, while the middle third consistently had the highest bone formation, bone formation at the apical third was smaller compared to the cervical third at the 8th week with this difference widening further at the 12th week.
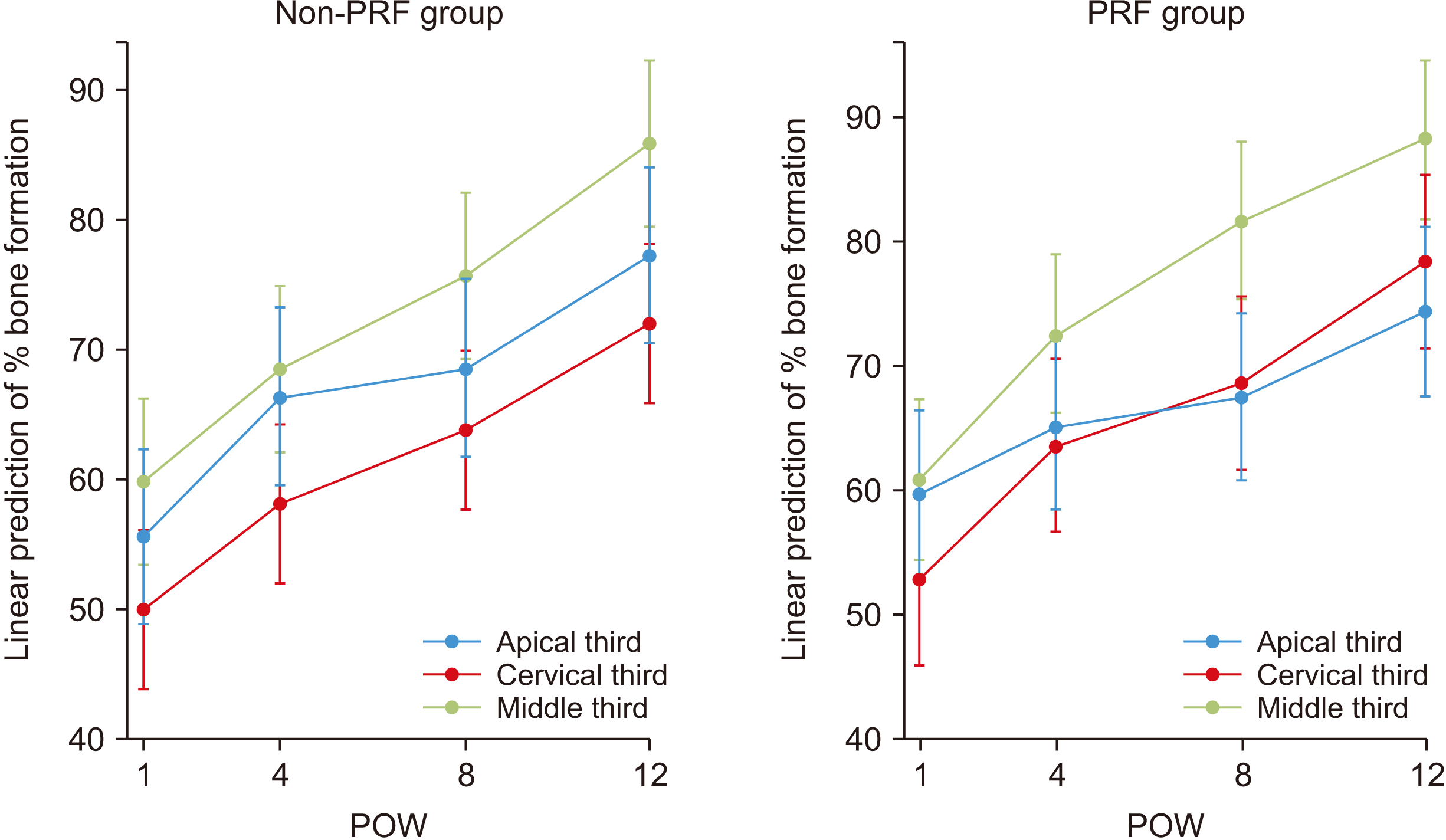 | Fig. 5Plot of linear prediction of bone formation following one-way MANOVA (multivariate ANOVA) of percentage bone formation on x-ray at cervical, middle, and apical portions of the tooth across postoperative follow-up measurement at weeks 1, 4, 8, and 12 for platelet-rich fibrin (PRF) and non-PRF groups. (POW: postoperative week) 
|
Table 3 shows the model output for the two-way MANOVA for bone formation at the cervical, middle, and apical portions of the tooth, and postoperative week and PRF group membership. There was a significant multivariate effect for percentage bone formation at the three locations as a group in relation to the timing of postoperative measurements and intervention group membership (Roy’s largest root=0.2652, F(4, 355)=23.53,
P<0.001). At the univariate level, there was a significant difference between bone formation across the four times of measurement (Roy’s largest root=0.2562, F(3, 355)= 30.32,
P<0.001). However, there was no significant difference in percentage bone formation between the intervention and control groups (Roy’s largest root=0.0149, F(3, 353)=1.75,
P=0.156).
Table 3
Two-way MANOVA (multivariate ANOVA) of percentage bone formation on the x-ray at the cervical, middle, and apical portions of the tooth across postoperative week and intervention group membership
|
Source |
Statistic |
df |
F (df1, df2) |
F |
P-value |
|
Model |
W=0.7828 |
4 |
12, 934.2 |
7.55 |
<0.001* |
|
P=0.2192 |
|
12, 1065 |
7.00 |
<0.001* |
|
L=0.2748 |
|
12, 1055 |
8.05 |
<0.001* |
|
R=0.2652 |
|
4, 355 |
23.53 |
<0.001* |
|
Residual |
|
355 |
|
|
|
|
Postoperative week |
W=0.7931 |
3 |
9, 859.3 |
9.54 |
<0.001* |
|
P=0.2077 |
|
9, 1065 |
8.80 |
<0.001* |
|
L=0.2600 |
|
9, 1055 |
10.16 |
<0.001* |
|
R=0.2562 |
|
3, 355 |
30.32 |
<0.001* |
|
Group |
W=0.9853 |
1 |
3, 353 |
1.75 |
0.156 |
|
P=0.0147 |
|
3, 353 |
1.75 |
0.156 |
|
L=0.0149 |
|
3, 353 |
1.75 |
0.156 |
|
R=0.0149 |
|
3, 353 |
1.75 |
0.156 |
|
Residual |
|
355 |
|
|
|
|
Total |
|
359 |
|
|
|

Table 4 shows the results of the comparison of mean percentage density of regions of newly formed bone (RNFB%) for the assessment of bone healing at different time points in the participants in the PRF and non-PRF groups. The RNFB% score was not significantly higher in the PRF group when compared with the non-PRF group at postoperative weeks 1, 4, 8, and 12 (
P=0.188, 0.155, 0.132, and 0.219, respectively). The RNFB% score was lowest at postoperative week 1 and highest at postoperative week 12 in both groups.
Table 4
Comparison of the mean percentage density of region of newly formed bone (RNFB%) for the assessment of bone healing at different time points in the PRF and non-PRF groups
|
POW |
Mean RNFB (%) |
P-value |
|
|
PRF group |
Non-PRF group |
|
1 |
57.74±9.39 |
54.98±10.33 |
0.188 |
|
4 |
67.07±7.92 |
64.24±10.58 |
0.155 |
|
8 |
77.70±9.02 |
74.25±11.60 |
0.132 |
|
12 |
85.95±6.73 |
83.97±7.90 |
0.219 |

Table 5 shows the regression coefficients from a mixed effect regression model separately for percentage bone formation on the x-ray at the cervical, middle, and apical portions of the tooth. There was lower average percentage bone formation among the PRF group compared with the non-PRF group at the cervical third (<1%), but higher percentages were observed at the middle (3.54%) and apical thirds (1.85%). However, these findings were not statistically significant. Compared with week 1, percentage bone formation was higher and increased from week 4 to week 12 for all of the cervical thirds (9.47%-23.81%;
P<0.001), middle thirds (10.24%-26.80%;
P<0.001), and apical thirds (8.19%-18.20%;
P<0.001). In addition, increase in percentage bone formation was the largest in the middle third for all of the 4th, 8th, and 12th week measurements.
Table 5
Mixed effect regression of percentage bone formation on the x-ray at the cervical, middle, and apical portions of the tooth with a robust estimation of variance, comparing intervention and control groups, and controlling for the duration of follow-ups and other demographic and clinical characteristics of the patients
|
Variable |
Cervical third |
|
Middle third |
|
Apical third |
|
|
|
|
B (95% CI) |
P-value |
B (95% CI) |
P-value |
B (95% CI) |
P-value |
|
Group |
|
|
|
|
|
|
|
Control |
|
|
|
|
|
|
|
Intervention |
–0.19 (–8.33 to 7.95) |
0.964 |
3.54 (–5.46 to 12.54) |
0.441 |
1.85 (–6.72 to 10.42) |
0.672 |
|
Postoperative week |
|
|
|
|
|
|
|
1 |
|
|
|
|
|
|
|
4 |
9.47 (5.84 to 13.11) |
<0.001* |
10.24 (7.12 to 13.36) |
<0.001* |
8.19 (4.17 to 12.2) |
<0.001* |
|
8 |
14.9 (9.09 to 20.71) |
<0.001* |
18.39 (12.65 to 24.14) |
<0.001* |
10.36 (4.72 to 16) |
<0.001* |
|
12 |
23.81 (17.61 to 30.02) |
<0.001* |
26.80 (20.63 to 32.98) |
<0.001* |
18.20 (12.45 to 23.96) |
<0.001* |
|
Age |
0.57 (–0.14 to 1.29) |
0.117 |
0.14 (–0.7 to 0.98) |
0.745 |
0.48 (–0.43 to 1.4) |
0.299 |
|
Sex |
|
|
|
|
|
|
|
Male |
|
|
|
|
|
|
|
Female |
4.28 (–2.4 to 10.96) |
0.209 |
2.83 (–5.09 to 10.74) |
0.484 |
7.04 (0.28 to 13.8) |
0.041* |
|
Local anaesthetic used |
–1.89 (–7.31 to 3.52) |
0.493 |
–2.06 (–8.4 to 4.28) |
0.524 |
–4.26 (–9.17 to 0.64) |
0.088 |
|
Impaction type |
|
|
|
|
|
|
|
Mesioangular |
|
|
|
|
|
|
|
Horizontal |
–10.79 (–21.23 to –0.36) |
0.043* |
–1.32 (–13.1 to 10.45) |
0.826 |
–3.42 (–13.5 to 6.66) |
0.507 |
|
Vertical |
–6.65 (–15.23 to 1.93) |
0.129 |
4.44 (–3.47 to 12.35) |
0.271 |
4.57 (–5.34 to 14.48) |
0.366 |
|
Distoangular |
–20.52 (–34.42 to –6.63) |
0.004* |
–4.25 (–16.3 to 7.81) |
0.490 |
5.32 (–7.36 to 18) |
0.411 |
|
Surgery duration |
0.37 (–0.16 to 0.89) |
0.170 |
–0.06 (–0.6 to 0.47) |
0.815 |
–0.59 (–1.08 to –0.1) |
0.018* |
|
Pederson |
–0.98 (–4.79 to 2.82) |
0.612 |
1.18 (–2.52 to 4.87) |
0.533 |
1.95 (–2.06 to 5.96) |
0.340 |
|
Associated pathology |
|
|
|
|
|
|
No pathology |
|
|
|
|
|
|
|
Pocket |
9.73 (–1.32 to 20.77) |
0.084 |
7.35 (–6.59 to 21.29) |
0.301 |
8.78 (–9.04 to 26.6) |
0.334 |
|
Pocket+caries |
4.76 (–7.65 to 17.17) |
0.452 |
3.49 (–12.47 to 19.45) |
0.668 |
2.77 (–16.56 to 22.11) |
0.779 |
|
Constant |
36.94 (5.54 to 68.33) |
0.021* |
50.4 (13.06 to 87.73) |
0.008* |
54.83 (14.04 to 95.61) |
0.008* |

The sexes of the patients were significantly associated with the percentage bone formation at the apical third with females having 7.04% more bone formation than males (P=0.041). The type of impaction was significantly associated with percentage bone formation at the cervical third with patients exhibiting horizontal and distoangular impactions having 10.79% (P=0.043) and 20.52% (P=0.004) less bone formation, respectively, compared with those that had mesioangular impactions. In addition, the duration of surgery was significantly associated with bone formation at the apical third with percentage bone formation reducing by 0.59% for every additional minute spent in surgery (P=0.018). Percentage bone formation at the cervical, middle, and apical portions were not significantly associated with the age of patient, Pederson score, and associated pathology.
Go to :

IV. Discussion
In this study, PRF was used as an autologous bone regenerative material and its effects were compared against the control group (non-PRF group). To our knowledge, no study in the literature has assessed the influence of age, sex, amount of local anesthetic used, impaction type, duration of surgery, Pederson difficulty score, and associated pathology on bone regeneration after mandibular third molar surgery. This study is also the first to assess the radiographic pattern of bone healing produced by PRF.
The aim of this study was to compare the effect of PRF with the control on extraction socket bone regeneration and the pattern of bone regeneration in the cervical, middle, and apical thirds of the extraction socket and the determinants of socket bone regeneration after the surgical extraction of impacted mandibular third molars.
In this study, grey scale was used to evaluate new bone formation using the HLImage++ software (ver. PCM: 18.0.3.q) (
Fig. 3) for the analysis of bone healing. This software has been validated for use for the measurement of new bone formation by previous studies
18,27. Several other studies
14,17 have employed the use of bone scintigraphy to assess bone healing. However, bone scintigraphy utilizes small amounts of radioactive material to diagnose bone diseases and is not sensitive for the diagnosis of new bone formation. In this study, new bone formation was monitored for 3 months postoperatively in support of the evidence that bone deposition in extraction sockets is greatest within the first three months after M3 extraction
28. This is similar to the follow-up period used to monitor the effect of PRF on bone healing in other studies
14,15,29.
There was no significant difference in percentage bone formation between the PRF and non-PRF groups in this study.(
Table 5) However, there was lower average percentage of bone formation among the PRF group compared with the non-PRF group at the cervical third (<1%), but was higher for the middle (3.54%) and apical thirds (1.85%). However, these findings were not statistically significant. Compared with week 1, the percentage of bone formation was higher and increased from week 4 to week 12 for all of the cervical third (9.47%-23.81%;
P<0.001), middle third (10.24%-28.80%;
P<0.001), and apical third (8.19%-18.20%;
P<0.001). In addition, the increase in percentage bone formation was the largest in the middle third for all of the 4th, 8th, and 12th week measurements. The percentage density of the RNFB (RNFB%) was higher even though it was not significant in the PRF group compared to the non-PRF group at all postoperative review visits.
This finding is supported by previous studies
14,15,17. Kumar et al.
15 reported non-significant higher bone density scores with radiography at three months postoperatively in the PRF group compared to the non-PRF group. Gürbüzer et al.
17 used scintigraphy to evaluate the effect of PRF on the early bone healing process after impacted M3 surgery. They reported that the average bone increase in technetium -99m methylene diphosphate uptake as an indicator of enhanced bone healing did not differ significantly between PRF treated and non-PRF treated sockets four weeks postoperatively. Baslarli et al.
14 used both scintigraphy and radiographic methods to determine the effect of PRF on bone healing after impacted M3 surgery with both methods showing no statistical evidence of greater bone regeneration in the PRF group. The reasons for insignificant increased bone healing in the extraction sockets of patients in the PRF group are speculative. Dohan Ehrenfest et al.
30 suggested a dose-dependent relationship between the quantity of PRF used and its ability to stimulate the proliferation and differentiation of oral BMSC (bone mesenchymal stem cells). This study has yet to be corroborated by other studies with higher evidence.
In contrast, Varghese et al.
18 reported a significant average percentage bone fill of 57.90±26.8 pixels in the PRF group and 46.74±17.7 pixels in the non-PRF group (
P=0.001). While the age range and tool for assessment of bone healing in the current study and that of Varghese et al.
18 are the same, their study was a split-mouth study and their bone evaluation was performed in the 1st, 4th, and 16th weeks postoperatively.
In the PRF group, while the middle third consistently exhibited the highest bone formation, bone formation at the apical third was smaller compared to the cervical third at the 8th week and this difference widened further at the 12th week. This peak in bone formation at the cervical third of the extraction socket may be attributed to the cumulative effects of the closer proximity of this region of the socket to the PRF clot. The PRF placed in the socket sits on the blood clot and is in closer proximity to the middle and cervical thirds of the extraction socket. Therefore, it is speculated that the concentration gradient of growth factors and cytokines in the middle and cervical regions of the extraction socket was greater compared to the apical region. However, this was not assessed in the present study. In the non-PRF group, the middle third consistently had the highest bone formation followed by the apical third, while the least bone formation was consistently observed in the cervical third.
The sex of the patients in this study was significantly associated with percentage bone formation at the apical third with females exhibiting 7.04% more bone formation compared to males (
P=0.041). This is similar to reports from Areewong et al.
31 where they conducted a prospective randomized clinical study involving thirty-six participants (18 participants in the PRF group and 18 participants in the control group) and reported increased bone formation (33.82±16.05 pixels vs 28.84±20.41 pixels) among females compared to males in the PRF group. However, their findings were not statistically significant (
P=0.573), possibly because of their limited sample size.
Variations in operating time are largely a reflection of the difficulty associated with the surgical removal of impacted M3s. In this study, there was a significant statistical difference between the mean duration of surgery in the PRF group (31.84±9.04 minutes) and non-PRF group (26.51±6.94 minutes). The difficulty index of impacted M3s in this study was not statistically significantly different between groups. Therefore, the difference in duration of surgery can only be attributed to the additional time needed for the insertion of PRF into the extraction sockets of the participants in the PRF group. The mean duration of surgery in the non-PRF group was 22.63 minutes, 25.0 minutes, and 21.92 minutes, respectively, as reported by Bello et al.
24, Sağlam et al.
32, and Rakprasitkul et al.
33. The duration of surgery in this study was significantly associated with bone formation at the apical third with percentage bone formation reduced by 0.59% for every additional minute spent in surgery (
P=0.018). This finding was supported by a study from Cheng et al.
34 were their meta-analyses demonstrated a 14% increase in the likelihood of complications including bone formation for every 30 minutes of additional operating time during bone surgery.
Go to :

V. Conclusion
The placement of PRF in extraction sockets after impacted M3 surgery improved socket bone regeneration. However, this finding was not statistically significant. In both groups, the middle third consistently exhibited the highest bone formation. The sex of the patient, type of impaction, and duration of surgery significantly influenced percentage of bone formation.
Go to :








 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download